Robust Plasma Cell Response to Skin-Inoculated Dengue Virus in Mice
- PMID: 33997054
- PMCID: PMC8096554
- DOI: 10.1155/2021/5511841
Robust Plasma Cell Response to Skin-Inoculated Dengue Virus in Mice
Abstract
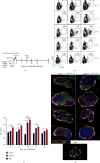
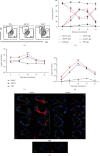
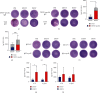
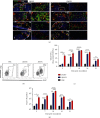
Dengue is a worldwide expanding threat caused by dengue virus (DENV) infection. To date, no specific treatment or effective vaccine is available. Antibodies produced by plasma cells (PCs) might be involved concomitantly in protection and severe dengue immunopathology. Although a massive appearance of PCs has been reported during acute DENV infection in humans, this response has been poorly characterized. Here, we show the dynamic of PC generation in immune-competent mice cutaneously inoculated with DENV compared with two control experimental groups: mice inoculated with inactivated DENV or with PBS. We found that PC numbers increased significantly in the skin-draining lymph node (DLN), peaking at day 10 and abruptly decreasing by day 14 after DENV inoculation. Class-switched IgG+ PCs appeared from day 7 and dominated the response, while in contrast, the frequency of IgM+ PCs decreased from day 7 onwards. Even though the kinetic of the response was similar between DENV- and iDENV-inoculated mice, the intensity of the response was significantly different. Interestingly, we demonstrated a similar PC response to virus antigens (E and prM) by ELISPOT. In situ characterization showed that PCs were distributed in the medullary cords and in close proximity to germinal centers (GCs), suggesting both an extrafollicular and a GC origin. Proliferating PCs (Ki-67+) were found as early as 3-day postinoculation, and in-depth analysis showed that these PCs were in active phases of cell cycle during the kinetic. Finally, we found a progressive appearance of high-affinity neutralizing DENV-specific IgG further supporting GC involvement. Of note, these antibodies seem to be highly cross-reactive, as a large proportion recognizes Zika virus (ZIKV). The strong PC response to skin-inoculated DENV in this work resembles the findings already described in humans. We consider that this study contributes to the understanding of the in vivo biology of the humoral immune response to DENV in an immunocompetent murine model.
Copyright © 2021 Raúl A. Maqueda-Alfaro et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures

Similar articles
-
Dengue and Zika virus infections are enhanced by live attenuated dengue vaccine but not by recombinant DSV4 vaccine candidate in mouse models.EBioMedicine. 2020 Oct;60:102991. doi: 10.1016/j.ebiom.2020.102991. Epub 2020 Sep 16. EBioMedicine. 2020. PMID: 32949997 Free PMC article.
-
Dengue and Zika Virus Domain III-Flagellin Fusion and Glycan-Masking E Antigen for Prime-Boost Immunization.Theranostics. 2019 Jul 9;9(16):4811-4826. doi: 10.7150/thno.35919. eCollection 2019. Theranostics. 2019. PMID: 31367259 Free PMC article.
-
Dengue Virus and Zika Virus Serological Cross-reactivity and Their Impact on Pathogenesis in Mice.J Infect Dis. 2019 Jan 7;219(2):223-233. doi: 10.1093/infdis/jiy482. J Infect Dis. 2019. PMID: 30085051
-
Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection.Front Immunol. 2018 Apr 23;9:597. doi: 10.3389/fimmu.2018.00597. eCollection 2018. Front Immunol. 2018. PMID: 29740424 Free PMC article. Review.
-
Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development.Front Immunol. 2019 Jun 11;10:1316. doi: 10.3389/fimmu.2019.01316. eCollection 2019. Front Immunol. 2019. PMID: 31244855 Free PMC article. Review.
References
-
- World Health Organization. Dengue and Severe Dengue. 2019. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

