Lower Gastrointestinal Bleeding Associated With Sodium Polystyrene Sulfonate Use
- PMID: 33997091
- PMCID: PMC8116012
- DOI: 10.14309/crj.0000000000000585
Lower Gastrointestinal Bleeding Associated With Sodium Polystyrene Sulfonate Use
Abstract
Sodium polystyrene sulfonate (kayexalate) is a cation-exchange resin widely used in the management of hyperkalemia. Gastrointestinal adverse events are uncommon; symptoms are nonspecific, and mucosal injury can range from mild ulceration to bowel perforation. An 81-year-old man was admitted because of decompensation of cirrhosis with acute kidney injury and hyperkalemia, treated with sodium polystyrene sulfonate. During admission, he presented multiple episodes of hematochezia, accompanied by tachycardia and hemoglobin drop. Colonoscopy revealed colonic ulceration, and histopathological findings were compatible with ulceration due to kayexalate injury. Despite rare, the widespread use of sodium polystyrene sulfonate puts a large population at risk of serious complications related to its use.
© 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Figures

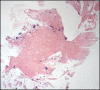
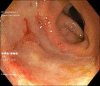
Similar articles
-
Kayexalate-Induced Esophageal Ulceration in a Patient with Decompensated Cirrhosis: A Review of the Literature.Case Rep Gastrointest Med. 2021 Feb 26;2021:8831814. doi: 10.1155/2021/8831814. eCollection 2021. Case Rep Gastrointest Med. 2021. PMID: 33728074 Free PMC article.
-
Intestinal Necrosis Associated with Orally Administered Calcium Polystyrene Sulfonate Without Sorbitol.Ann Pharmacother. 2011 Feb;45(2):e13. doi: 10.1345/aph.1M547. Ann Pharmacother. 2011. PMID: 21304040
-
Rectal ulcer due to Kayexalate deposition - an unusual case.Rev Assoc Med Bras (1992). 2018 Aug;64(8):680-683. doi: 10.1590/1806-9282.64.08.680. Rev Assoc Med Bras (1992). 2018. PMID: 30673037
-
Colonic necrosis in a young patient receiving oral kayexalate in sorbitol: case report and literature review.Kaohsiung J Med Sci. 2011 Apr;27(4):155-8. doi: 10.1016/j.kjms.2010.12.010. Epub 2011 Feb 16. Kaohsiung J Med Sci. 2011. PMID: 21463839 Free PMC article. Review.
-
Hyperkalemia and potential pitfalls of sodium polystyrene sulfonate.JAAPA. 2015 Mar;28(3):41-5. doi: 10.1097/01.JAA.0000458856.92020.1e. JAAPA. 2015. PMID: 25710403 Review.
Cited by
-
Hyperkalemia: Pharmacotherapies and Clinical Considerations.Cureus. 2024 Jan 26;16(1):e52994. doi: 10.7759/cureus.52994. eCollection 2024 Jan. Cureus. 2024. PMID: 38406030 Free PMC article. Review.
References
-
- Kessler C, Ng J, Valdez K, Xie H, Geiger B. SPS in hyperkalemia. J Hosp Med 2011;3:136–40. - PubMed
-
- Cheng ES, Stringer KM, Pegg SP. Colonic necrosis and perforation following oral sodium polystyrene sulfonate (resonium A/kayexalate) in a burn patient. Burns 2002;28:189–90. - PubMed
-
- Lillemoe KD, Romolo JL, Hamilton SR, Pennington LR, Burdick JF, Williams GM. Intestinal necrosis due to sodium polystyrene (kayexalate) in sorbitol enemas: Clinical and experimental support for the hypothesis. Surgery 1987;101:267–72. - PubMed
-
- Gerstman BB, Kirkman R, Platt R. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. Am J Kidney Dis 1992;20(2):159–61. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

