Genetically blocking HPD via CRISPR-Cas9 protects against lethal liver injury in a pig model of tyrosinemia type I
- PMID: 33997102
- PMCID: PMC8099604
- DOI: 10.1016/j.omtm.2021.04.002
Genetically blocking HPD via CRISPR-Cas9 protects against lethal liver injury in a pig model of tyrosinemia type I
Abstract
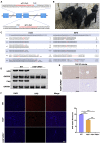
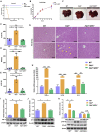
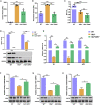
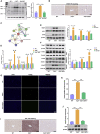
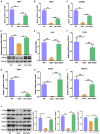
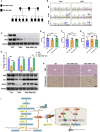
Hereditary tyrosinemia type I (HT1) results from the loss of fumarylacetoacetate hydrolase (FAH) activity and can lead to lethal liver injury (LLI). Therapeutic options for HT1 remain limited. The FAH -/- pig, a well-characterized animal model of HT1, represents a promising candidate for testing novel therapeutic approaches to treat this condition. Here, we report an improved single-step method to establish a biallelic (FAH -/- ) mutant porcine model using CRISPR-Cas9 and cytoplasmic microinjection. We also tested the feasibility of rescuing HT1 pigs through inactivating the 4-hydroxyphenylpyruvic acid dioxygenase (HPD) gene, which functions upstream of the pathogenic pathway, rather than by directly correcting the disease-causing gene as occurs with traditional gene therapy. Direct intracytoplasmic delivery of CRISPR-Cas9 targeting HPD before intrauterine death reprogrammed the tyrosine metabolism pathway and protected pigs against FAH deficiency-induced LLI. Characterization of the F1 generation revealed consistent liver-protective features that were germline transmissible. Furthermore, HPD ablation ameliorated oxidative stress and inflammatory responses and restored the gene profile relating to liver metabolism homeostasis. Collectively, this study not only provided a novel large animal model for exploring the pathogenesis of HT1, but also demonstrated that CRISPR-Cas9-mediated HPD ablation alleviated LLI in HT1 pigs and represents a potential therapeutic option for the treatment of HT1.
Keywords: CRISPR/Cas9; HPD ablation; lethal liver injury; liver hemeostasis; metabolic reprogramming; oxidative stress; pig; tyrosinemia type I.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Gagné R. [Genetic counseling: experience of 4 years] Union Med. Can. 1978;107:391–393. - PubMed
-
- Jorquera R., Tanguay R.M. Fumarylacetoacetate, the metabolite accumulating in hereditary tyrosinemia, activates the ERK pathway and induces mitotic abnormalities and genomic instability. Hum. Mol. Genet. 2001;10:1741–1752. - PubMed
-
- Bliksrud Y.T., Ellingsen A., Bjørås M. Fumarylacetoacetate inhibits the initial step of the base excision repair pathway: implication for the pathogenesis of tyrosinemia type I. J. Inherit. Metab. Dis. 2013;36:773–778. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

