VPS33B suppresses lung adenocarcinoma metastasis and chemoresistance to cisplatin
- PMID: 33997178
- PMCID: PMC8093570
- DOI: 10.1016/j.gendis.2019.12.009
VPS33B suppresses lung adenocarcinoma metastasis and chemoresistance to cisplatin
Abstract
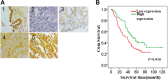
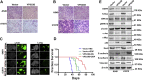
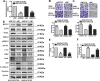
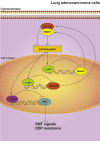
The presence of VPS33B in tumors has rarely been reported. Downregulated VPS33B protein expression is an unfavorable factor that promotes the pathogenesis of lung adenocarcinoma (LUAD). Overexpressed VPS33B was shown to reduce the migration, invasion, metastasis, and chemoresistance of LUAD cells to cisplatin (DDP) in vivo and in vitro. Mechanistic analyses have indicated that VPS33B first suppresses epidermal growth factor receptor (EGFR) Ras/ERK signaling, which further reduces the expression of the oncogenic factor c-Myc. Downregulated c-Myc expression reduces the rate at which it binds the p53 promoter and weakens its transcription inhibition; therefore, decreased c-Myc stimulates p53 expression, leading to decreased epithelial-to-mesenchymal transition (EMT) signal. NESG1 has been shown to be an unfavorable indicator of non-small-cell lung cancer (NSCLC). Here, NESG1 was identified as an interactive protein of VPS33B. In addition, NESG1 was found to exhibit mutual stimulation with VPS33B via reduced RAS/ERK/c-Jun-mediated transcription repression. Knockdown of NESG1 activated EGFR/Ras/ERK/c-Myc signaling and further downregulated p53 expression, which thus activated EMT signaling and promoted LUAD migration and invasion. Finally, we observed that nicotine suppressed VPS33B expression by inducing PI3K/AKT/c-Jun-mediated transcription suppression. Our study demonstrates that VPS33B as a tumor suppressor is significantly involved in the pathogenesis of LUAD.
Keywords: Chemoresistance; Lung adenocarcinoma; Metastasis; Nicotine; VPS33B.
© 2020 Chongqing Medical University. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare no conflict of interests.
Figures



References
-
- Cafarotti S., Lococo F., Froesh P., Zappa F., Andre D. Target therapy in lung cancer. Adv Exp Med Biol. 2016;893:127–136. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

