Protective effect of nano vitamin D against fatty degeneration in submandibular and sublingual salivary glands: A histological and ultrastructural study
- PMID: 33997429
- PMCID: PMC8102766
- DOI: 10.1016/j.heliyon.2021.e06932
Protective effect of nano vitamin D against fatty degeneration in submandibular and sublingual salivary glands: A histological and ultrastructural study
Abstract
Background: Poor nutritional habits and a low level of physical activity are associated with obesity, leading to increased caloric and fat intakes. A high-fat diet can significantly impact oral health through the accumulation of lipids in the salivary glands, which ultimately affect salivary gland function. Recently, an increasing number of supplement nano-formulations, such as nano vitamin D, have become available. However, only few studies have explored the effects of nano vitamin D on the maintenance of oral health.
Objective: This study aimed to compare the histological effects of nano vitamin D to those of regular vitamin D on fatty degeneration in submandibular and sublingual salivary glands using a rat model.
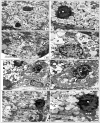
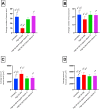
Methods: Twenty-four adult male albino Sprague-Dawley rats were divided into the following groups: untreated group, high-fat diet group, high-fat diet and regular vitamin D group, and high-fat diet and nano vitamin group.Thereafter, samples of the submandibular and sublingual salivary glands were dissected for histological and electron microscopic studies. Morphometric digital image analysis was used to quantitatively measure the changes in the size and number of acini and secretory granules.
Results: Regular vitamin D had a partial protective effect. However, vitamin D could fully restore cellular structures to their normal state, thereby protecting against fatty degeneration of the salivary tissue and immune cell infiltration, particularly in the submandibular serous tissue. Nano vitamin D was more efficacious than regular vitamin D at restoring the number and size of submandibular serous secretory granules.
Conclusion: Employing nano vitamin D as a supplement to high-fat diets could protect against high-fat diet-induced salivary gland damage in rats.
Keywords: Degeneration; High-fat diet; Histology; Nano vitamin D; Rat; Salivary gland; Sublingual; Submandibular.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Early Alterations of Cytoskeletal Proteins Induced by Radiation Therapy in the Parenchymal Cells of Rat Major Salivary Glands: A Comparative Immunohistochemical Analysis.Cureus. 2024 Dec 13;16(12):e75634. doi: 10.7759/cureus.75634. eCollection 2024 Dec. Cureus. 2024. PMID: 39803043 Free PMC article.
-
The effect of essential fatty acid deficiency on the fatty acid composition of different salivary glands and saliva in rats.Arch Oral Biol. 1997 Oct-Nov;42(10-11):727-34. doi: 10.1016/s0003-9969(97)00071-x. Arch Oral Biol. 1997. PMID: 9447262
-
A comparative quantitative histological investigation of atrophic changes in the major salivary glands of liquid-fed rats.Arch Oral Biol. 1991;36(11):855-7. doi: 10.1016/0003-9969(91)90035-s. Arch Oral Biol. 1991. PMID: 1763982
-
Characterization of common salivary protein 1, a product of rat submandibular, sublingual, and parotid glands.J Biol Chem. 1993 Dec 15;268(35):26592-601. J Biol Chem. 1993. PMID: 8253789
-
Immunohistochemical Evaluation of the Pathological Effects of Diabetes Mellitus on the Major Salivary Glands of Albino Rats.Eur J Dent. 2023 May;17(2):485-491. doi: 10.1055/s-0042-1749159. Epub 2022 Jul 4. Eur J Dent. 2023. PMID: 35785821 Free PMC article.
Cited by
-
Prophylactic effect of nano vitamin D3 against fatty degeneration caused by calcium-deficient diet in parotid salivary gland: a histological and ultrastructural study.BMC Oral Health. 2025 Jul 30;25(1):1279. doi: 10.1186/s12903-025-06618-7. BMC Oral Health. 2025. PMID: 40739560 Free PMC article.
References
-
- Kurek K., Piotrowska D.M., Wiesiołek-Kurek P., Łukaszuk B., Chabowski A., Górski J., Żendzian-Piotrowska M. Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver Int. 2014;34:1074–1083. - PubMed
-
- Bonen A., Tandon N., Glatz J., Luiken J., Heigenhauser G. The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int. J. Obes. 2006;30:877–883. - PubMed
-
- Hand A., Weiss R.E. Effects of streptozotocin-induced diabetes on the rat parotid gland. Lab. Invest. J. Tech. Methods Pathol. 1984;51:429–440. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

