Sand-mediated ice seeding enables serum-free low-cryoprotectant cryopreservation of human induced pluripotent stem cells
- PMID: 33997514
- PMCID: PMC8111032
- DOI: 10.1016/j.bioactmat.2021.04.025
Sand-mediated ice seeding enables serum-free low-cryoprotectant cryopreservation of human induced pluripotent stem cells
Abstract
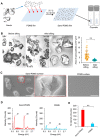
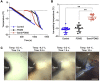
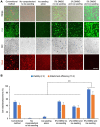
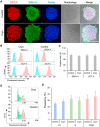
Human induced pluripotent stem cells (hiPSCs) possess tremendous potential for tissue regeneration and banking hiPSCs by cryopreservation for their ready availability is crucial to their widespread use. However, contemporary methods for hiPSC cryopreservation are associated with both limited cell survival and high concentration of toxic cryoprotectants and/or serum. The latter may cause spontaneous differentiation and/or introduce xenogeneic factors, which may compromise the quality of hiPSCs. Here, sand from nature is discovered to be capable of seeding ice above -10 °C, which enables cryopreservation of hiPSCs with no serum, much-reduced cryoprotectant, and high cell survival. Furthermore, the cryopreserved hiPSCs retain high pluripotency and functions judged by their pluripotency marker expression, cell cycle analysis, and capability of differentiation into the three germ layers. This unique sand-mediated cryopreservation method may greatly facilitate the convenient and ready availability of high-quality hiPSCs and probably many other types of cells/tissues for the emerging cell-based translational medicine.
Keywords: Cryopreservation; Ice seeding; Sand; Stem cell; iPSC.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Freezing Responses in DMSO-Based Cryopreservation of Human iPS Cells: Aggregates Versus Single Cells.Tissue Eng Part C Methods. 2018 May;24(5):289-299. doi: 10.1089/ten.TEC.2017.0531. Epub 2018 Mar 28. Tissue Eng Part C Methods. 2018. PMID: 29478388 Free PMC article.
-
A Protocol for Culture and Characterization of Human Induced Pluripotent Stem Cells After Induction.Curr Protoc. 2023 Aug;3(8):e866. doi: 10.1002/cpz1.866. Curr Protoc. 2023. PMID: 37610273 Free PMC article.
-
Cell Banking of hiPSCs: A Practical Guide to Cryopreservation and Quality Control in Basic Research.Curr Protoc Stem Cell Biol. 2020 Dec;55(1):e127. doi: 10.1002/cpsc.127. Curr Protoc Stem Cell Biol. 2020. PMID: 32956561
-
Cryopreservation: Vitrification and Controlled Rate Cooling.Methods Mol Biol. 2017;1590:41-77. doi: 10.1007/978-1-4939-6921-0_5. Methods Mol Biol. 2017. PMID: 28353262 Review.
-
Principles of cryopreservation by vitrification.Methods Mol Biol. 2015;1257:21-82. doi: 10.1007/978-1-4939-2193-5_2. Methods Mol Biol. 2015. PMID: 25428002 Review.
Cited by
-
Overcoming ice: cutting-edge materials and advanced strategies for effective cryopreservation of biosample.J Nanobiotechnology. 2025 Mar 7;23(1):187. doi: 10.1186/s12951-025-03265-6. J Nanobiotechnology. 2025. PMID: 40050919 Free PMC article. Review.
-
Pollen derived macromolecules serve as a new class of ice-nucleating cryoprotectants.Sci Rep. 2022 Jul 19;12(1):12295. doi: 10.1038/s41598-022-15545-4. Sci Rep. 2022. PMID: 35854036 Free PMC article.
-
Controlled Ice Nucleation With a Sand-PDMS Film Device Enhances Cryopreservation of Mouse Preantral Ovarian Follicles.J Med Device. 2024 Dec 1;18(4):041007. doi: 10.1115/1.4066445. Epub 2024 Sep 30. J Med Device. 2024. PMID: 39465055
-
A highly active mineral-based ice nucleating agent supports in situ cell cryopreservation in a high throughput format.J R Soc Interface. 2023 Feb;20(199):20220682. doi: 10.1098/rsif.2022.0682. Epub 2023 Feb 8. J R Soc Interface. 2023. PMID: 36751925 Free PMC article.
-
The Effect of Short- and Long-Term Cryopreservation on Chicken Primordial Germ Cells.Genes (Basel). 2024 May 14;15(5):624. doi: 10.3390/genes15050624. Genes (Basel). 2024. PMID: 38790253 Free PMC article.
References
-
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., Slukvin I.I., Thomson J.A. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

