Carrier-free highly drug-loaded biomimetic nanosuspensions encapsulated by cancer cell membrane based on homology and active targeting for the treatment of glioma
- PMID: 33997516
- PMCID: PMC8111096
- DOI: 10.1016/j.bioactmat.2021.04.027
Carrier-free highly drug-loaded biomimetic nanosuspensions encapsulated by cancer cell membrane based on homology and active targeting for the treatment of glioma
Abstract
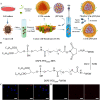
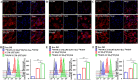
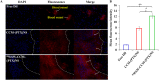
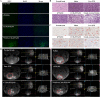
Nanosuspensions, as a new drug delivery system for insoluble drugs, are only composed of a drug and a small amount of stabilizer, which is dispersed in an aqueous solution with high drug-loading, small particle size, high dispersion, and large specific surface area. It can significantly improve the dissolution, bioavailability, and efficacy of insoluble drugs. In this study, paclitaxel nanosuspensions ((PTX)NS) were prepared by an ultrasonic precipitation method, with the characteristics of simple preparation and easy repetition. With the help of a homologous targeting mechanism, a kind of glioma C6 cancer cell membrane (CCM)-coated (PTX)NS was developed and modified with DWSW peptide to obtain DWSW-CCM-(PTX)NS with the functions of BBB penetration and tumor targeting. The results showed that the cancer cell membrane could effectively camouflage the nanosuspensions so that it was not cleared by the immune system and could cross the blood-brain-barrier (BBB) and selectively target tumor tissues. Cell uptake experiments and in vivo imaging confirmed that the uptake of DWSW-CCM-(PTX)NS by tumor cells and the distribution in intracranial gliomas increased. Cytotoxicity test and in vivo anti-glioma studies showed that DWSW-CCM-(PTX)NS could significantly inhibit the growth of glioma cells and significantly prolong the survival time of glioma-bearing mice. Finally, the cancer cell membrane coating endowed the nanosuspensions with the biological properties of homologous adhesion and immune escape. This study provides an integrated solution for improving the targeting of nanosuspensions and demonstrates the encouraging potential of biomimetic nanosuspensions applicable to tumor therapy.
Keywords: Biomimetic drug-delivery systems; Blood-brain barrier; Cancer cell membrane; Gliomas; Nanosuspensions; Paclitaxel.
© 2021 The Authors.
Conflict of interest statement
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work. There is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of the manuscript entitled “Carrier-free Highly Drug-loaded Biomimetic Nanosuspensions Encapsulated by Cancer Cell Membrane Based on Homology and Active Targeting for the Treatment of Glioma”.
Figures





References
-
- Wang N., Cheng X., Li N., Wang H., Chen H. Nanocarriers and their loading strategies. Adv Healthc Mater. 2019;8(6) e1801002. - PubMed
-
- Rabinow B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004;3(9):785–796. - PubMed
-
- Patel D., Zode S.S., Bansal A.K. Formulation aspects of intravenous nanosuspensions. Int. J. Pharm. 2020;586:119555. - PubMed
-
- Chai Z., Ran D., Lu L., Zhan C., Ruan H., Hu X., Xie C., Jiang K., Li J., Zhou J., Wang J., Zhang Y., Fang R.H., Zhang L., Lu W. Ligand-modified cell membrane enables the targeted delivery of drug nanocrystals to glioma. ACS Nano. 2019;13(5):5591–5601. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

