Renal Outcomes in Patients with Systolic Heart Failure Treated With Sacubitril-Valsartan or Angiotensin Converting Enzyme Inhibitor/Angiotensin Receptor Blocker
- PMID: 33997628
- PMCID: PMC8105557
- DOI: 10.1016/j.mayocpiqo.2020.10.008
Renal Outcomes in Patients with Systolic Heart Failure Treated With Sacubitril-Valsartan or Angiotensin Converting Enzyme Inhibitor/Angiotensin Receptor Blocker
Abstract
Objective: To assess 4 adverse renal outcomes in a heterogeneous cohort of patients with systolic heart failure (HF) who were prescribed sacubitril-valsartan vs angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEi/ARB).
Patients and methods: The OptumLabs Database Warehouse, which contains linked administrative claims and laboratory results, was used to identify patients with systolic HF who were prescribed sacubitril-valsartan or ACEi/ARB between July 1, 2015, and September 30, 2019. One-to-one propensity score matching and inverse probability of treatment weighting was used to balance baseline variables. Cox proportional hazards modeling was performed to compare renal outcomes in both medication groups, including 30% or more decline in estimated glomerular filtration rate (eGFR), doubling of serum creatinine, acute kidney injury (AKI), and kidney failure (eGFR < 15 mL/min per 1.73 m2, kidney transplant, or dialysis initiation).
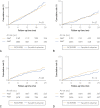
Results: A total of 4667 matched pairs receiving sacubitril-valsartan or ACEi/ARB were included; the mean follow-up period was 7.8±7.8 months. The mean age was 69.4±11 years; 35% were female, 19% black, and 15% Hispanic. The cumulative risk at 1 year was 6% for 30% or more decline in eGFR, 2% for doubling of serum creatinine, 3% for AKI, and 2% to 3% for kidney failure. Furthermore, no significant differences in risk were observed with sacubitril-valsartan compared with ACEi/ARB for a 30% or more decline in eGFR (hazard ratio [HR], 0.96; 95% CI, 0.79 to 1.10), doubling of serum creatinine (HR, 0.94; 95% CI, 0.69 to 1.27); AKI (HR, 0.80; 95% CI, 0.63 to 1.03), and kidney failure (HR 0.80; 95% CI, 0.59 to 1.08).
Conclusion: Among patients with systolic HF, the risk of adverse renal outcomes was similar between patients prescribed sacubitril-valsartan and those prescribed ACEi/ARB.
Keywords: ACEi, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; ICD-10, International Classification of Diseases, Tenth Revision; ICD-9, International Classification of Diseases, Ninth Revision; IPTW, inverse probability of treatment weighting; NP, natriuretic peptide; RAAS, renin-angiotensin-aldosterone system; RCT, randomized controlled trial; eGFR, estimated glomerular filtration rate.
© 2020 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc.
Figures


References
-
- Verbrugge F.H., Tang W.H.W., Mullens W. Renin-angiotensin-aldosterone system activation during decongestion in acute heart failure: friend or foe? JACC Heart Fail. 2015;3(2):108–111. - PubMed
-
- Yancy C.W., Jessup M., Bozkurt B., et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America [published correction appears in J Am Coll Cardiol. 2016;68(13):1495] J Am Coll Cardiol. 2016;68(13):1476–1488. - PubMed
-
- SOLVD Investigators. Yusuf S., Pitt B., Davis C.E., Hood W.B., Cohn J.N. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. - PubMed
-
- Cohn J.N., Tognoni G., Valsartan Heart Failure Trial Invetsigators A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667–1675. - PubMed
-
- Schefold J.C., Filippatos G., Hasenfuss G., Anker S.D., von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12(10):610–623. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

