Boosting mitochondria activity by silencing MCJ overcomes cholestasis-induced liver injury
- PMID: 33997750
- PMCID: PMC8099785
- DOI: 10.1016/j.jhepr.2021.100276
Boosting mitochondria activity by silencing MCJ overcomes cholestasis-induced liver injury
Abstract
Background & aims: Mitochondria are the major organelles for the formation of reactive oxygen species (ROS) in the cell, and mitochondrial dysfunction has been described as a key factor in the pathogenesis of cholestatic liver disease. The methylation-controlled J-protein (MCJ) is a mitochondrial protein that interacts with and represses the function of complex I of the electron transport chain. The relevance of MCJ in the pathology of cholestasis has not yet been explored.
Methods: We studied the relationship between MCJ and cholestasis-induced liver injury in liver biopsies from patients with chronic cholestatic liver diseases, and in livers and primary hepatocytes obtained from WT and MCJ-KO mice. Bile duct ligation (BDL) was used as an animal model of cholestasis, and primary hepatocytes were treated with toxic doses of bile acids. We evaluated the effect of MCJ silencing for the treatment of cholestasis-induced liver injury.
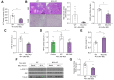
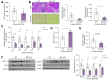
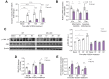
Results: Elevated levels of MCJ were detected in the liver tissue of patients with chronic cholestatic liver disease when compared with normal liver tissue. Likewise, in mouse models, the hepatic levels of MCJ were increased. After BDL, MCJ-KO animals showed significantly decreased inflammation and apoptosis. In an in vitro model of bile-acid induced toxicity, we observed that the loss of MCJ protected mouse primary hepatocytes from bile acid-induced mitochondrial ROS overproduction and ATP depletion, enabling higher cell viability. Finally, the in vivo inhibition of the MCJ expression, following BDL, showed reduced liver injury and a mitigation of the main cholestatic characteristics.
Conclusions: We demonstrated that MCJ is involved in the progression of cholestatic liver injury, and our results identified MCJ as a potential therapeutic target to mitigate the liver injury caused by cholestasis.
Lay summary: In this study, we examine the effect of mitochondrial respiratory chain inhibition by MCJ on bile acid-induced liver toxicity. The loss of MCJ protects hepatocytes against apoptosis, mitochondrial ROS overproduction, and ATP depletion as a result of bile acid toxicity. Our results identify MCJ as a potential therapeutic target to mitigate liver injury in cholestatic liver diseases.
Keywords: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMA-M2, antimitochondrial M2 antibody; ANA, antinuclear antibodies; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; Abs, antibodies; BA, bile acid; BAX, BCL2 associated X; BCL-2, B-cell lymphoma 2; BCL-Xl, B-cell lymphoma-extra large; BDL, bile duct ligation; Bile duct ligation; CLD, cholestatic liver disease; Ccl2, C-C motif chemokine ligand 2; Ccr2, C-C motif chemokine receptor 2; Ccr5, C-C motif chemokine receptor 5; Cholestasis; Cxcl1, C-X-C motif chemokine ligand 1; Cyp7α1, cholesterol 7 alpha-hydroxylase; DCA, deoxycholic acid; ETC, electron transport chain; Ezh2, enhancer of zeste homolog 2; Fxr, farnesoid X receptor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GCDCA, glycochenodeoxycholic acid; HSC, hepatic stellate cells; Hif-1α, hypoxia-inducible factor 1-alpha; JNK, c-Jun N-terminal kinase; KO, knockout; LSM, liver stiffness; MAPK, mitogen-activated protein kinase; MCJ; MCJ, methylation-controlled J; MLKL, mixed-lineage kinase domain-like pseudokinase; MMP, mitochondrial membrane potential; MPO, myeloperoxidase; MPT, mitochondrial permeability transition; Mitochondria; Nrf1, nuclear respiratory factor 1; PARP, poly (ADP-ribose) polymerase; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; Pgc1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; Pgc1β, peroxisome proliferator-activated receptor gamma coactivator 1-beta; ROS; ROS, reactive oxygen species; RT, room temperature; SDH2, succinate dehydrogenase; TNF, tumour necrosis factor; Tfam, transcription factor A mitochondrial; Trail, TNF-related apoptosis-inducing ligand; UDCA, ursodeoxycholic acid; Ucp2, uncoupling protein 2; VCTE, vibration-controlled transient elastography; WT, wild-type; mRNA, messenger ribonucleic acid; p-JNK, phosphor-JNK; p-MLKL, phosphor-MLKL; shRNA, small hairpin RNA; siRNA, small interfering RNA; tBIL, total bilirubin; α-SMA, alpha-smooth muscle actin.
© 2021 The Author(s).
Conflict of interest statement
Dr. María Luz Martínez-Chantar advises for Mitotherapeutix LLC. Dr. Javier Crespo reports grants and research support from Gilead Sciences, AbbVie, MSD, and Intercept Pharmaceuticals (all outside the scope of the submitted work). He is a speaker for Gilead Sciences and AbbVie. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- Hirschfield G.M., Beuers U., Corpechot C., Invernizzi P., Jones D., Marzioni M. EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145–172. - PubMed
-
- Nevens F., Andreone P., Mazzella G., Strasser S.I., Bowlus C., Invernizzi P. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375:631–643. - PubMed
-
- Jansen P.L.M., Ghallab A., Vartak N., Reif R., Schaap F.G., Hampe J. The ascending pathophysiology of cholestatic liver disease. Hepatology. 2017;65:722–738. - PubMed
-
- Saito J.M., Maher J.J. Bile duct ligation in rats induces biliary expression of cytokine-induced neutrophil chemoattractant. Gastroenterology. 2000;118:1157–1168. - PubMed
-
- Gujral J.S., Farhood A., Bajt M.L., Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

