DNA damage measurements within tissue samples with Repair Assisted Damage Detection (RADD)
- PMID: 33997769
- PMCID: PMC8118132
- DOI: 10.1016/j.crbiot.2019.11.001
DNA damage measurements within tissue samples with Repair Assisted Damage Detection (RADD)
Abstract
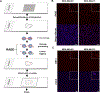
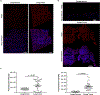
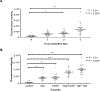
Exposures to genotoxic carcinogens and reactive species result in strand breaks and a spectrum of covalent modifications to DNA that can induce mutations and contribute to the initiation and progression of cancer. Measurements of DNA damage within tissue or tumor samples can serve as a biomarker for exposures or assess changes in DNA repair capacity relevant in cancer development and treatment. Numerous methods to characterize DNA damage exist. However, these methods are primarily applicable to isolated DNA or cultured cells, often require a substantial amount of material, and may be limited to the detection and quantification of only a handful of DNA adducts. Here, we used the Repair Assisted Damage Detection (RADD) assay to detect and excise DNA adducts using a cocktail of DNA repair enzymes, then the damage site within the genome are tagged for detection using a modified nucleotide. We previously demonstrated the RADD assay can detect lesions within isolated DNA and fixed cells, and now RADD can be used to detect DNA adducts and DNA strand breaks in formalin-fixed paraffin-embedded (FFPE) tissue samples. We verified the ability of the RADD assay to detect DNA damage in tissue by exogenously inducing DNA damage with X-rays and restriction enzymes. We also showed that RADD can be multiplexed with antibodies to detect cell cycle markers or other proteins of interest. Finally, we showed that RADD can detect DNA damage within clinically relevant ovarian tumor samples. RADD is a flexible and easy-to-use assay that allows relative damage levels to be determined within FFPE samples and allows the heterogeneity of DNA adducts and strand breaks within clinically relevant samples to be measured.
Conflict of interest statement
Declaration of competing interests The authors declare no conflicts of interest.
Figures

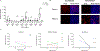
References
-
- Olive PL, Banáth JP: The comet assay: a method to measure DNA damage in individual cells. Nat Protoc 2006, 1:23–29. - PubMed
-
- Smith CC, O’Donovan MR, Martin EA: hOGG1 recognizes oxidative damage using the comet assay with greater specificity than FPG or ENDOIII. Mutagenesis 2006, 21:185–190. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
