Ion-channel regulation of response decorrelation in a heterogeneous multi-scale model of the dentate gyrus
- PMID: 33997798
- PMCID: PMC7610774
- DOI: 10.1016/j.crneur.2021.100007
Ion-channel regulation of response decorrelation in a heterogeneous multi-scale model of the dentate gyrus
Abstract
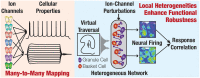
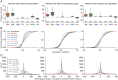
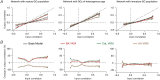
Heterogeneities in biological neural circuits manifest in afferent connectivity as well as in local-circuit components such as neuronal excitability, neural structure and local synaptic strengths. The expression of adult neurogenesis in the dentate gyrus (DG) amplifies local-circuit heterogeneities and guides heterogeneities in afferent connectivity. How do neurons and their networks endowed with these distinct forms of heterogeneities respond to perturbations to individual ion channels, which are known to change under several physiological and pathophysiological conditions? We sequentially traversed the ion channels-neurons-network scales and assessed the impact of eliminating individual ion channels on conductance-based neuronal and network models endowed with disparate local-circuit and afferent heterogeneities. We found that many ion channels differentially contributed to specific neuronal or network measurements, and the elimination of any given ion channel altered several functional measurements. We then quantified the impact of ion-channel elimination on response decorrelation, a well-established metric to assess the ability of neurons in a network to convey complementary information, in DG networks endowed with different forms of heterogeneities. Notably, we found that networks constructed with structurally immature neurons exhibited functional robustness, manifesting as minimal changes in response decorrelation in the face of ion-channel elimination. Importantly, the average change in output correlation was dependent on the eliminated ion channel but invariant to input correlation. Our analyses suggest that neurogenesis-driven structural heterogeneities could assist the DG network in providing functional resilience to molecular perturbations.
Keywords: Adult neurogenesis; Channel decorrelation; Computational model; Heterogeneities hippocampus; Intrinsic plasticity; Ion channels; Multiscale analysis.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Ion-channel degeneracy and heterogeneities in the emergence of complex spike bursts in CA3 pyramidal neurons.J Physiol. 2023 Aug;601(15):3297-3328. doi: 10.1113/JP283539. Epub 2022 Oct 23. J Physiol. 2023. PMID: 36201674 Free PMC article.
-
Disparate forms of heterogeneities and interactions among them drive channel decorrelation in the dentate gyrus: Degeneracy and dominance.Hippocampus. 2019 Apr;29(4):378-403. doi: 10.1002/hipo.23035. Epub 2018 Dec 7. Hippocampus. 2019. PMID: 30260063 Free PMC article.
-
Dominant role of adult neurogenesis-induced structural heterogeneities in driving plasticity heterogeneity in dentate gyrus granule cells.Hippocampus. 2022 Jul;32(7):488-516. doi: 10.1002/hipo.23422. Epub 2022 May 13. Hippocampus. 2022. PMID: 35561083 Free PMC article.
-
Contributions of adult neurogenesis to dentate gyrus network activity and computations.Behav Brain Res. 2019 Nov 18;374:112112. doi: 10.1016/j.bbr.2019.112112. Epub 2019 Aug 1. Behav Brain Res. 2019. PMID: 31377252 Free PMC article. Review.
-
Regulation of adult-born and mature neurons in stress response and antidepressant action in the dentate gyrus of the hippocampus.Neurosci Res. 2025 Feb;211:10-15. doi: 10.1016/j.neures.2022.08.010. Epub 2022 Aug 27. Neurosci Res. 2025. PMID: 36030966 Review.
Cited by
-
Plasticity manifolds and degeneracy govern circadian oscillations of neuronal intrinsic properties in the suprachiasmatic nucleus.iScience. 2023 Mar 27;26(4):106503. doi: 10.1016/j.isci.2023.106503. eCollection 2023 Apr 21. iScience. 2023. PMID: 37123240 Free PMC article.
-
Ion-channel degeneracy: Multiple ion channels heterogeneously regulate intrinsic physiology of rat hippocampal granule cells.Physiol Rep. 2021 Aug;9(15):e14963. doi: 10.14814/phy2.14963. Physiol Rep. 2021. PMID: 34342171 Free PMC article.
-
Active Dendrites and Local Field Potentials: Biophysical Mechanisms and Computational Explorations.Neuroscience. 2022 May 1;489:111-142. doi: 10.1016/j.neuroscience.2021.08.035. Epub 2021 Sep 8. Neuroscience. 2022. PMID: 34506834 Free PMC article. Review.
-
Degeneracy in epilepsy: multiple routes to hyperexcitable brain circuits and their repair.Commun Biol. 2023 May 3;6(1):479. doi: 10.1038/s42003-023-04823-0. Commun Biol. 2023. PMID: 37137938 Free PMC article. Review.
-
Ion-channel degeneracy and heterogeneities in the emergence of complex spike bursts in CA3 pyramidal neurons.J Physiol. 2023 Aug;601(15):3297-3328. doi: 10.1113/JP283539. Epub 2022 Oct 23. J Physiol. 2023. PMID: 36201674 Free PMC article.
References
-
- Aimone J.B., Gage F.H. Modeling new neuron function: a history of using computational neuroscience to study adult neurogenesis. Eur. J. Neurosci. 2011;33:1160–1169. - PubMed
-
- Aimone J.B., Wiles J., Gage F.H. Potential role for adult neurogenesis in the encoding of time in new memories. Nat. Neurosci. 2006;9:723–727. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

