The effect of targeting Tie2 on hemorrhagic shock-induced renal perfusion disturbances in rats
- PMID: 33997943
- PMCID: PMC8126531
- DOI: 10.1186/s40635-021-00389-5
The effect of targeting Tie2 on hemorrhagic shock-induced renal perfusion disturbances in rats
Abstract
Background: Hemorrhagic shock is associated with acute kidney injury and increased mortality. Targeting the endothelial angiopoietin/Tie2 system, which regulates endothelial permeability, previously reduced hemorrhagic shock-induced vascular leakage. We hypothesized that as a consequence of vascular leakage, renal perfusion and function is impaired and that activating Tie2 restores renal perfusion and function.
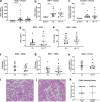
Methods: Rats underwent 1 h of hemorrhagic shock and were treated with either vasculotide or PBS as control, followed by fluid resuscitation for 4 h. Microcirculatory perfusion was measured in the renal cortex and cremaster muscle using contrast echography and intravital microscopy, respectively. Changes in the angiopoietin/Tie2 system and renal injury markers were measured in plasma and on protein and mRNA level in renal tissue. Renal edema formation was determined by wet/dry weight ratios and renal structure by histological analysis.
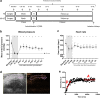
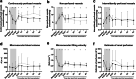
Results: Hemorrhagic shock significantly decreased renal perfusion (240 ± 138 to 51 ± 40, p < 0.0001) and cremaster perfusion (12 ± 2 to 5 ± 2 perfused vessels, p < 0.0001) compared to baseline values. Fluid resuscitation partially restored both perfusion parameters, but both remained below baseline values (renal perfusion 120 ± 58, p = 0.08, cremaster perfusion 7 ± 2 perfused vessels, p < 0.0001 compared to baseline). Hemorrhagic shock increased circulating angiopoietin-1 (p < 0.0001), angiopoietin-2 (p < 0.0001) and soluble Tie2 (p = 0.05), of which angiopoietin-2 elevation was associated with renal edema formation (r = 0.81, p < 0.0001). Hemorrhagic shock induced renal injury, as assessed by increased levels of plasma neutrophil gelatinase-associated lipocalin (NGAL: p < 0.05), kidney injury marker-1 (KIM-1; p < 0.01) and creatinine (p < 0.05). Vasculotide did not improve renal perfusion (p > 0.9 at all time points) or reduce renal injury (NGAL p = 0.26, KIM-1 p = 0.78, creatinine p > 0.9, renal edema p = 0.08), but temporarily improved cremaster perfusion at 3 h following start of fluid resuscitation compared to untreated rats (resuscitation + 3 h: 11 ± 3 vs 8 ± 3 perfused vessels, p < 0.05).
Conclusion: Hemorrhagic shock-induced renal impairment cannot be restored by standard fluid resuscitation, nor by activation of Tie2. Future treatment strategies should focus on reducing angiopoietin-2 levels or on activating Tie2 via an alternative strategy.
Keywords: Acute kidney injury; Contrast-enhanced ultrasonography; Endothelium; Hemorrhage; Microcirculatory perfusion; Vascular leakage.
Conflict of interest statement
PVS is listed as inventor on multiple patents related to Vasculotide filed by Sunnybrook Health Sciences Centre and Vasomune Therapeutics, Canada. For the remaining authors none were declared.
Figures

References
-
- Hutchings SD, Naumann DN, Hopkins P, et al. Microcirculatory impairment is associated with multiple organ dysfunction following traumatic hemorrhagic shock: The MICROSHOCK Study. Crit Care Med. 2018;46(9):e889–e896. - PubMed
-
- Harrois A, Libert N, Duranteau J. Acute kidney injury in trauma patients. Curr Opin Crit Care. 2017;23(6):447–456. - PubMed
-
- Tachon G, Harrois A, Tanaka S, et al. Microcirculatory alterations in traumatic hemorrhagic shock. Crit Care Med. 2014;42(6):1433–1441. - PubMed
Grants and funding
- 2016T064/Hartstichting
- Prof Heimburger Award/CSL Behring Foundation for Research and Advancement of Patient Health
- Research project grant 2016/European Society of Anaesthesiology
- Levi-Montalcini Award 2017/European Society of Intensive Care Medicine
- Young Investigator Grant 2017/Dutch society of anesthesiology
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

