Preparation of Butadienylpyridines by Iridium-NHC-Catalyzed Alkyne Hydroalkenylation and Quinolizine Rearrangement
- PMID: 33998070
- PMCID: PMC8453560
- DOI: 10.1002/chem.202101414
Preparation of Butadienylpyridines by Iridium-NHC-Catalyzed Alkyne Hydroalkenylation and Quinolizine Rearrangement
Abstract
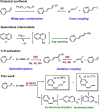
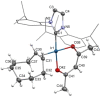
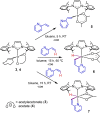
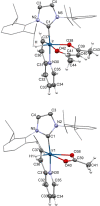
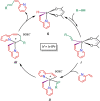
Iridium(I) N-heterocyclic carbene complexes of formula Ir(κ2 O,O'-BHetA)(IPr)(η2 -coe) [BHetA=bis-heteroatomic acidato, acetylacetonate or acetate; IPr=1,3-bis(2,6-diisopropylphenyl)imidazolin-2-carbene; coe=cyclooctene] have been prepared by treating Ir(κ2 O,O'-BHetA)(η2 -coe)2 complexes with IPr. These complexes react with 2-vinylpyridine to afford the hydrido-iridium(III)-alkenyl cyclometalated derivatives IrH(κ2 O,O'-BHetA)(κ2 N,C-C7 H6 N)(IPr) through the iridium(I) intermediate Ir(κ2 O,O'-BHetA)(IPr)(η2 -C7 H7 N). The cyclometalated IrH(κ2 O,O'-acac)(κ2 N,C-C7 H6 N)(IPr) complex efficiently catalyzes the hydroalkenylation of aromatic and aliphatic terminal alkynes and enynes with 2-vinylpyridine to afford 2-(4R-butadienyl)pyridines with Z,E configuration as the major reaction products (yield up to 89 %). In addition, unprecedented (Z)-2-butadienyl-5R-pyridine derivatives have been obtained as minor reaction products (yield up to 21 %) from the elusive 1Z,3gem-butadienyl hydroalkenylation products. These compounds undergo a thermal 6π-electrocyclization to afford bicyclic 4H-quinolizine derivatives that, under catalytic reaction conditions, tautomerize to 6H-quinolizine to afford the (Z)-2-(butadienyl)-5R-pyridine by a retro-electrocyclization reaction.
Keywords: C−C coupling; C−H activation; N-heterocyclic carbenes; catalysis; hydroalkenylation; iridium.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- None
-
- Kakiuchi F., Murai S., Acc. Chem. Res. 2002, 35, 826–834; - PubMed
-
- Wencel-Delord J., Droge T., Liu F., Glorius F., Chem. Soc. Rev. 2011, 40, 4740–4761; - PubMed
-
- Wang J.-P., Gan L., Liu Y., Org. Biomol. Chem. 2017, 15, 9031–9043; - PubMed
-
- Wang C.-S., Dixneuf P. H., Soulé J.-F., Chem. Rev. 2018, 118, 7532–7585; - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

