Automated Left Ventricular Dimension Assessment Using Artificial Intelligence Developed and Validated by a UK-Wide Collaborative
- PMID: 33998247
- PMCID: PMC8136463
- DOI: 10.1161/CIRCIMAGING.120.011951
Automated Left Ventricular Dimension Assessment Using Artificial Intelligence Developed and Validated by a UK-Wide Collaborative
Abstract
Background: requires training and validation to standards expected of humans. We developed an online platform and established the Unity Collaborative to build a dataset of expertise from 17 hospitals for training, validation, and standardization of such techniques.
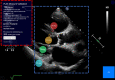
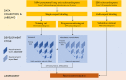
Methods: The training dataset consisted of 2056 individual frames drawn at random from 1265 parasternal long-axis video-loops of patients undergoing clinical echocardiography in 2015 to 2016. Nine experts labeled these images using our online platform. From this, we trained a convolutional neural network to identify keypoints. Subsequently, 13 experts labeled a validation dataset of the end-systolic and end-diastolic frame from 100 new video-loops, twice each. The 26-opinion consensus was used as the reference standard. The primary outcome was precision SD, the SD of the differences between AI measurement and expert consensus.
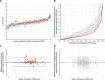
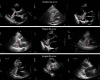
Results: In the validation dataset, the AI's precision SD for left ventricular internal dimension was 3.5 mm. For context, precision SD of individual expert measurements against the expert consensus was 4.4 mm. Intraclass correlation coefficient between AI and expert consensus was 0.926 (95% CI, 0.904-0.944), compared with 0.817 (0.778-0.954) between individual experts and expert consensus. For interventricular septum thickness, precision SD was 1.8 mm for AI (intraclass correlation coefficient, 0.809; 0.729-0.967), versus 2.0 mm for individuals (intraclass correlation coefficient, 0.641; 0.568-0.716). For posterior wall thickness, precision SD was 1.4 mm for AI (intraclass correlation coefficient, 0.535 [95% CI, 0.379-0.661]), versus 2.2 mm for individuals (0.366 [0.288-0.462]). We present all images and annotations. This highlights challenging cases, including poor image quality and tapered ventricles.
Conclusions: Experts at multiple institutions successfully cooperated to build a collaborative AI. This performed as well as individual experts. Future echocardiographic AI research should use a consensus of experts as a reference. Our collaborative welcomes new partners who share our commitment to publish all methods, code, annotations, and results openly.
Keywords: consensus; echocardiography; hospital; left ventricle; machine learning.
Figures
References
-
- Popescu BA, Stefanidis A, Nihoyannopoulos P, Fox KF, Ray S, Cardim N, Rigo F, Badano LP, Fraser AG, Pinto F, et al. . Updated standards and processes for accreditation of echocardiographic laboratories from The European Association of Cardiovascular Imaging: an executive summary. Eur Heart J Cardiovasc Imaging. 2014;15:1188–1193. doi: 10.1093/ehjci/jeu057 - PubMed
-
- Yu KH, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719–731. doi: 10.1038/s41551-018-0305-z - PubMed
-
- Bulat A, Tzimiropoulos G. Human pose estimation via convolutional part heatmap regression. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). 2016. Springer Nature: vol. 9911 LNCS. Springer Verlag. 717–732. doi: 10.1007/978-3-319-46478-7_44
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

