DACH1 protects podocytes from experimental diabetic injury and modulates PTIP-H3K4Me3 activity
- PMID: 33998601
- PMCID: PMC8121508
- DOI: 10.1172/JCI141279
DACH1 protects podocytes from experimental diabetic injury and modulates PTIP-H3K4Me3 activity
Abstract
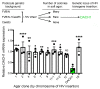
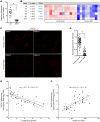
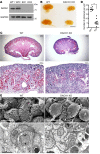
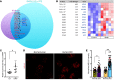
Dachshund homolog 1 (DACH1), a key cell-fate determinant, regulates transcription by DNA sequence-specific binding. We identified diminished Dach1 expression in a large-scale screen for mutations that convert injury-resistant podocytes into injury-susceptible podocytes. In diabetic kidney disease (DKD) patients, podocyte DACH1 expression levels are diminished, a condition that strongly correlates with poor clinical outcomes. Global Dach1 KO mice manifest renal hypoplasia and die perinatally. Podocyte-specific Dach1 KO mice, however, maintain normal glomerular architecture at baseline, but rapidly exhibit podocyte injury after diabetes onset. Furthermore, podocyte-specific augmentation of DACH1 expression in mice protects from DKD. Combined RNA sequencing and in silico promoter analysis reveal conversely overlapping glomerular transcriptomic signatures between podocyte-specific Dach1 and Pax transactivation-domain interacting protein (Ptip) KO mice, with upregulated genes possessing higher-than-expected numbers of promoter Dach1-binding sites. PTIP, an essential component of the activating histone H3 lysine 4 trimethylation (H3K4Me3) complex, interacts with DACH1 and is recruited by DACH1 to its promoter-binding sites. DACH1-PTIP recruitment represses transcription and reduces promoter H3K4Me3 levels. DACH1 knockdown in podocytes combined with hyperglycemia triggers target gene upregulation and increases promoter H3K4Me3. These findings reveal that in DKD, diminished DACH1 expression enhances podocyte injury vulnerability via epigenetic derepression of its target genes.
Keywords: Chronic kidney disease; Diabetes; Epigenetics; Nephrology.
Conflict of interest statement
Figures





Comment in
-
DACH1 as a multifaceted and potentially druggable susceptibility factor for kidney disease.J Clin Invest. 2021 May 17;131(10):e149043. doi: 10.1172/JCI149043. J Clin Invest. 2021. PMID: 33998596 Free PMC article.
-
DACH1 protects podocytes and tubules from injury.Nat Rev Nephrol. 2021 Aug;17(8):511. doi: 10.1038/s41581-021-00450-2. Nat Rev Nephrol. 2021. PMID: 34083770 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

