Lacosamide add-on therapy for focal epilepsy
- PMID: 33998660
- PMCID: PMC8127139
- DOI: 10.1002/14651858.CD008841.pub3
Lacosamide add-on therapy for focal epilepsy
Abstract
Background: This is an updated version of the Cochrane review published in 2015. Around half of people with epilepsy will not achieve seizure freedom on their first antiepileptic drug; many will require add-on therapy. Around a third of people fail to achieve complete seizure freedom despite multiple antiepileptic drugs. Lacosamide has been licenced as an add-on therapy for drug-resistant focal epilepsy.
Objectives: To evaluate the efficacy and tolerability of lacosamide as an add-on therapy for children and adults with drug-resistant focal epilepsy.
Search methods: We searched the following databases (22 August 2019): the Cochrane Register of Studies (CRS Web), including the Cochrane Epilepsy Group Specialized Register and the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid, 1946 to 20 August 2019), ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform (ICTRP), with no language restrictions. We contacted UCB Pharma (sponsors of lacosamide).
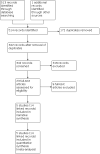
Selection criteria: Randomised controlled trials of add-on lacosamide in people with drug-resistant focal epilepsy.
Data collection and analysis: We used standard Cochrane methodology, assessing the following outcomes: 50% or greater reduction in seizure frequency; seizure freedom; treatment withdrawal; adverse events; quality of life; and cognitive changes. The primary analyses were intention-to-treat. We estimated summary risk ratios (RR) for each outcome presented with 99% confidence intervals (CI), except for 50% or greater seizure reduction, seizure freedom and treatment withdrawal which were presented with 95% CIs. We performed subgroup analyses according to lacosamide dose and sensitivity analyses according to population age, whereby data from children were excluded from the meta-analysis.
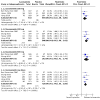
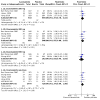
Main results: We included five trials (2199 participants). The risk of bias for all studies was low to unclear. All studies were placebo-controlled and assessed doses from 200 mg to 600 mg per day. One study evaluated lacosamide in children; all other studies were in adults. Trial duration ranged from 24 to 26 weeks. All studies used adequate methods of randomisation and were double-blind. Overall, the certainty of the evidence for the outcomes was judged as moderate to high, with the exception of seizure freedom which was low. The RR for a 50% or greater reduction in seizure frequency for all doses of lacosamide compared with placebo was 1.79 (95% CI 1.55 to 2.08; 5 studies; 2199 participants; high-certainty evidence). The RR for seizure freedom for all doses of lacosamide compared with placebo was 2.27 (95% CI 1.35 to 3.83; 5 studies; 2199 participants; low-certainty evidence). The RR for treatment withdrawal for all doses of lacosamide compared with placebo was 1.57 (95% CI 1.24 to 1.98; 5 studies; 2199 participants; moderate-certainty evidence). The estimated effect size for most outcomes did not change considerably following sensitivity analysis. For seizure freedom, however, the RR nearly doubled upon the exclusion of data from children (RR 4.04, 95% CI 1.52 to 10.73). Adverse events associated with lacosamide included: abnormal co-ordination (RR 6.12, 99% CI 1.35 to 27.77), blurred vision (RR 4.65, 99% CI 1.24 to 17.37), diplopia (RR 5.59, 99% CI 2.27 to 13.79), dizziness (RR 2.96, 99% CI 2.09 to 4.20), nausea (RR 2.35, 99% CI 1.37 to 4.02), somnolence (RR 2.04, 99% CI 1.22 to 3.41), vomiting (RR 2.94, 99% CI 1.54 to 5.64), and number of participants experiencing one or more adverse events (RR 1.12, 99% CI 1.01 to 1.24). Adverse events that were not significant were: vertigo (RR 3.71, 99% CI 0.86 to 15.95), rash (RR 0.58, 99% CI 0.17 to 1.89), nasopharyngitis (RR 1.41, 99% CI 0.87 to 2.28), headache (RR 1.34, 99% CI 0.90 to 1.98), fatigue (RR 2.11, 99% CI 0.92 to 4.85), nystagmus (RR 1.47, 99% CI 0.61 to 3.52), and upper respiratory tract infection (RR 0.70, 99% CI 0.43 to 1.15).
Authors' conclusions: Lacosamide is effective and well-tolerated in the short term when used as add-on treatment for drug-resistant focal epilepsy. Lacosamide increases the number of people with 50% or greater reduction in seizure frequency and may increase seizure freedom, compared to placebo. Higher doses of lacosamide may be associated with higher rates of adverse events and treatment withdrawal. Additional evidence is required assessing the use of lacosamide in children and on longer-term efficacy and tolerability.
Trial registration: ClinicalTrials.gov NCT01921205.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
RKB: none known RB: none known CSG: none known BDM has received funding from the National Institute for Health Research, Medical Research Council, Wellcome Trust, Academy of Medical Sciences, and British Medical Association. No others known.
Figures
























Update of
-
Lacosamide add-on therapy for partial epilepsy.Cochrane Database Syst Rev. 2015 Jun 16;(6):CD008841. doi: 10.1002/14651858.CD008841.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2021 May 17;5:CD008841. doi: 10.1002/14651858.CD008841.pub3. PMID: 26077821 Updated.
References
References to studies included in this review
Ben Menachem 2007 {published and unpublished data}
-
- Ben Menachem E, Biton V, Jatuzis D, Abou-Khalil B, Doty P, Rudd D, SP 667 Study Group. Efficacy and safety of adjunctive oral lacosamide for the treatment of partial onset seizures in patients with epilepsy. Epilepsia 2005;46(Suppl 6):57, Abstract no: 030.
-
- Ben Menachem E. Lacosamide - efficacy and tolerability [abstract]. Epilepsia 2006;47(Suppl 3):272.
Chung 2010 {published and unpublished data}
-
- Chung S, Sperling M, Biton V, Krauss G, Beaman M, Hebert D. Efficacy and safety of lacosamide as an adjunctive treatment for partial-onset seizures. Epilepsia 2009;50(Suppl 4):110, Abstract no: T231.
-
- Chung S, Sperling M, Biton V, Krauss G, Doty P, Sullivan T. Lacosamide: efficacy and safety as oral adjunctive therapy in adults with partial onset seizures. Epilepsia 2007;48(Suppl 7):57, Abstract no: 065. - PubMed
-
- Chung SS, Sperling M, Biton V, Krauss G, Beaman M, Hebert D. Lacosamide: efficacy and safety as oral adjunctive treatment for partial onset seizures. Epilepsia 2007;48(Suppl 6):321, Abstract no: 3.197.
Halasz 2009 {published and unpublished data}
-
- Halász P, Kälviäinen R, Mazurkiewicz-Beldzińska M, Rosenow F, Doty P, Hebert D, et al, SP755 Study Group. Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia 2009;50(3):443-53. [DOI: 10.1111/j.1528-1167.2008.01951.x] [PMID: ] - DOI - PubMed
-
- Halasz P, Kalviainen R, Mazurkiewicz-Beldzinska M, Rosenow F, Doty P, Sullivan T. Lacosamide: efficacy and safety as oral adjunctive therapy in adults with partial seizures. Epilepsia 2006;47(Suppl 4):3, Abstract no: A.07. - PubMed
Hong 2016 {published data only}
-
- Hong Z, Inoue Y, Liao W, Meng H, Wang X, Wang W, et al. Efficacy and safety of adjunctive lacosamide for the treatment of partial-onset seizures in Chinese and Japanese adults: a randomized, double-blind, placebo-controlled study. Epilepsy Research 2016;127:267-75. [DOI: 10.1016/j.eplepsyres.2016.08.032] [PMID: ] - DOI - PubMed
NCT01921205 {unpublished data only}
-
- Farkas V, Steinborn B, Flamini J, Dilley D, Bozorg A, Daniels T, et al. Efficacy and tolerability of adjunctive lacosamide in children and adolescents with uncontrolled focal seizures: a randomized, double-blind, placebo-controlled trial. Annals of Neurology 2017;82(Suppl 21):S287-90, Abstract no: 36.
-
- Farkas V, Steinborn B, Flamini J, Zhang Y, Yuen N, Borghs S, et al. Tolerability and efficacy of adjunctive lacosamide in children and adolescents with focal seizures in context of presence or absence of sodium-channel blocking AEDs: post-hoc analysis of a randomized, double-blind, placebo-controlled trial. Neurology 2018;90(15 Suppl):Abstract no: P5.260.
-
- NCT01921205. Study to investigate lacosamide as add-on therapy in subjects ≥4 years to <17 years of age with partial onset seizures [Study to evaluate the efficacy of lacosamide (LCM) administered in addition to 1 to ≤3 other anti-epileptic drugs in subjects with epilepsy ≥4 years to <17 years of age who currently have uncontrolled partial onset seizures]. clinicaltrials.gov/ct2/show/NCT01921205 (first received 19 March 2018).
-
- Steinborn B, Stockis A, Zhang Y, Dimova S, Martin P. Lack of pharmacokinetic interaction of lacosamide on carbamazepine, lamotrigine, levetiracetam, oxcarbazepine, topiramate and valproic acid in children and adolescents with epilepsy: post-hoc analysis of a randomized, double-blind, placebo-controlled trial. Neurology 2018;90(15 Suppl):Abstract no: P4.270.
References to studies excluded from this review
Biton 2008 {published data only}
-
- Biton V, Rosenfeld W, Mameniskiene R, Vaiciene N, Whitesides J, Sommerville K. Safety and tolerability of intravenous lacosamide as replacement for oral lacosamide in subjects with partial-onset seizures. Epilepsia 2005;46(Suppl 6):120-1, Abstract no: p258.
Ferreira 2019 {published data only}
Grosso 2014 {published data only}
Jatuzis 2006 {published data only}
-
- Jatuzis D, Biton V, Ben-Menachem E, Abou-Khalil B, Doty P, Rudd D. Evaluation of the effect of oral lacosamide on concomitant AED plasma concentrations in patients with partial seizures. Epilepsia 2005;46(Suppl 8):170, Abstract no: 2.236.
-
- Jatuzis D, Biton V, Ben-Menachem E, Abou-Khalil B, Hackenson M, Rudd D. Evaluation of the effect of oral lacosamide on adjunctive concomitant antiepileptic drug plasma concentrations in subjects with partial seizures. Epilepsia 2006;47(Suppl 3):145-6, Abstract no: p561.
Kalviainen 2007 {published data only}
-
- Kalviainen R, Halasz P, Mazurkiewicz-Beldinska M, Rosenow F, Hopeken B, Rudd G. Lacosamide in subjects with partial-onset seizures: evaluation of the effect of oral lacosamide on concomitant antiepileptic drug plasma concentrations. Epilepsia 2007;48(Suppl 7):57, Abstract no: 066.
NCT01724918 {unpublished data only}
-
- NCT01724918. Lacosamide IV and EEG/EKG (LIVE) study (LIVE) [Lacosamide IV and EEG/EKG (LIVE) study (LIVE)]. clinicaltrials.gov/ct2/show/NCT01724918 (first received 12 November 2012).
Rosenfeld 2005 {published data only}
-
- Rosenfeld W, Biton V, Mameniskiene R, Vaiciene N, Whitesides J, Schiltmeyer B, et al. Pharmacokinetics and safety of intravenous lacosamide administered as replacement for adjunctive oral lacosamide in patients with partial-onset seizures. Epilepsia 2005;46(Suppl 8):184, Abstract no: 2.278.
Additional references
Beghi 2019
Brigo 2016
-
- Brigo F, Bragazzi N, Nardone R, Trinka E. Efficacy and tolerability of brivaracetam compared to lacosamide, eslicarbazepine acetate, and perampanel as adjunctive treatments in uncontrolled focal epilepsy: results of an indirect comparison meta-analysis of RCTs. Seizure 2016;42:29-37. [DOI: 10.1016/j.seizure.2016.08.007] [PMID: ] - DOI - PubMed
Chen 2016
Doty 2007
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 12 March 2021. Available at gradepro.org.
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011).
Higgins 2021
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. - PMC - PubMed
Kellinghaus 2009
Kirkham 2010
Kirkham 2018
Kwan 2000
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. New England Journal of Medicine 2000;342(5):314-9. [PMID: ] - PubMed
Kwan 2010
-
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Hauser WA, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51(6):1069-77. - PubMed
Lefebvre 2019
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6 (updated October 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/supplementary-materials.
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Reynolds 1981
-
- Reynolds E, Shorvon SD, Galbraith AW, Chadwick D, Dellaportas CI, Vydelingum L. Phenytoin for epilepsy: a long term prospective study, assisted by serum level monitoring, in previously untreated patients. Epilepsia 1981;22(4):475-88. [PMID: ] - PubMed
Rosenow 2016
Scheffer 2017
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
References to other published versions of this review
Shukralla 2010
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

