Antibiotic regimens for late-onset neonatal sepsis
- PMID: 33998665
- PMCID: PMC8127057
- DOI: 10.1002/14651858.CD013836.pub2
Antibiotic regimens for late-onset neonatal sepsis
Abstract
Background: Neonatal sepsis is a major cause of morbidity and mortality. It is the third leading cause of neonatal mortality globally constituting 13% of overall neonatal mortality. Despite the high burden of neonatal sepsis, high-quality evidence in diagnosis and treatment is scarce. Due to the diagnostic challenges of sepsis and the relative immunosuppression of the newborn, many neonates receive antibiotics for suspected sepsis. Antibiotics have become the most used therapeutics in neonatal intensive care units, and observational studies in high-income countries suggest that 83% to 94% of newborns treated with antibiotics for suspected sepsis have negative blood cultures. The last Cochrane Review was updated in 2005. There is a need for an updated systematic review assessing the effects of different antibiotic regimens for late-onset neonatal sepsis.
Objectives: To assess the beneficial and harmful effects of different antibiotic regimens for late-onset neonatal sepsis.
Search methods: We searched the following electronic databases: CENTRAL (2021, Issue 3); Ovid MEDLINE; Embase Ovid; CINAHL; LILACS; Science Citation Index EXPANDED and Conference Proceedings Citation Index - Science on 12 March 2021. We also searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi-RCTs.
Selection criteria: We included RCTs comparing different antibiotic regimens for late-onset neonatal sepsis. We included participants older than 72 hours of life at randomisation, suspected or diagnosed with neonatal sepsis, meningitis, osteomyelitis, endocarditis, or necrotising enterocolitis. We excluded trials that assessed treatment of fungal infections.
Data collection and analysis: Three review authors independently assessed studies for inclusion, extracted data, and assessed risk of bias. We used the GRADE approach to assess the certainty of evidence. Our primary outcome was all-cause mortality, and our secondary outcomes were: serious adverse events, respiratory support, circulatory support, nephrotoxicity, neurological developmental impairment, necrotising enterocolitis, and ototoxicity. Our primary time point of interest was at maximum follow-up.
Main results: We included five RCTs (580 participants). All trials were at high risk of bias, and had very low-certainty evidence. The five included trials assessed five different comparisons of antibiotics. We did not conduct a meta-analysis due to lack of relevant data. Of the five included trials one trial compared cefazolin plus amikacin with vancomycin plus amikacin; one trial compared ticarcillin plus clavulanic acid with flucloxacillin plus gentamicin; one trial compared cloxacillin plus amikacin with cefotaxime plus gentamicin; one trial compared meropenem with standard care (ampicillin plus gentamicin or cefotaxime plus gentamicin); and one trial compared vancomycin plus gentamicin with vancomycin plus aztreonam. None of the five comparisons found any evidence of a difference when assessing all-cause mortality, serious adverse events, circulatory support, nephrotoxicity, neurological developmental impairment, or necrotising enterocolitis; however, none of the trials were near an information size that could contribute significantly to the evidence of the comparative benefits and risks of any particular antibiotic regimen. None of the trials assessed respiratory support or ototoxicity. The benefits and harms of different antibiotic regimens remain unclear due to the lack of well-powered trials and the high risk of systematic errors.
Authors' conclusions: Current evidence is insufficient to support any antibiotic regimen being superior to another. RCTs assessing different antibiotic regimens in late-onset neonatal sepsis with low risks of bias are warranted.
Antecedentes: La sepsis neonatal es una causa importante de morbilidad y mortalidad. Es la tercera causa de mortalidad neonatal a nivel mundial y constituye el 13% de la mortalidad neonatal total. A pesar de la elevada carga de la sepsis neonatal, la evidencia de alta calidad en el diagnóstico y el tratamiento es escasa. Debido a las dificultades de diagnóstico de la sepsis y a la relativa inmunosupresión del neonato, muchos reciben antibióticos por sospecha de sepsis. Los antibióticos se han convertido en el tratamiento más utilizado en las unidades de cuidados intensivos neonatales, y los estudios observacionales realizados en países de ingresos altos indican que entre el 83% y el 94% de los neonatos tratados con antibióticos por sospecha de sepsis tienen hemocultivos negativos. La última revisión Cochrane se actualizó en 2005. Se necesita una revisión sistemática actualizada que evalúe los efectos de los diferentes regímenes de antibióticos para la sepsis neonatal de inicio tardío.
Objetivos: Evaluar los efectos beneficiosos y perjudiciales de diferentes regímenes antibióticos para la sepsis neonatal de inicio tardío. MÉTODOS DE BÚSQUEDA: Se hicieron búsquedas en las siguientes bases de datos electrónicas: CENTRAL (2021, número 3); Ovid MEDLINE; Embase Ovid; CINAHL; LILACS; Science Citation Index EXPANDED y Conference Proceedings Citation Index ‐ Science el 12 de marzo de 2021. También se buscaron ensayos controlados aleatorizados (ECA) y cuasialeatorizados en las bases de datos de ensayos clínicos y en las listas de referencias de artículos identificados. CRITERIOS DE SELECCIÓN: Se incluyeron ECA que compararon diferentes regímenes de antibióticos para la sepsis neonatal de inicio tardío. Se incluyeron participantes mayores de 72 horas de vida en el momento de la asignación al azar, con sospecha o diagnóstico de sepsis neonatal, meningitis, osteomielitis, endocarditis o enterocolitis necrosante. Se excluyeron los ensayos que evaluaron el tratamiento de las infecciones micóticas. OBTENCIÓN Y ANÁLISIS DE LOS DATOS: Dos autores de la revisión, de forma independiente, evaluaron los estudios para inclusión, extrajeron los datos y evaluaron el riesgo de sesgo. Se utilizó el método GRADE para evaluar la certeza de la evidencia. El desenlace principal fue la mortalidad por todas las causas, y los desenlaces secundarios fueron: eventos adversos graves, asistencia respiratoria, apoyo circulatorio, nefrotoxicidad, deterioro del desarrollo neurológico, enterocolitis necrosante y ototoxicidad. El punto temporal principal de interés fue el seguimiento máximo.
Resultados principales: Se incluyeron cinco ECA (580 participantes). Todos los ensayos tuvieron alto riesgo de sesgo y evidencia de certeza muy baja. Los cinco ensayos incluidos evaluaron cinco comparaciones diferentes de antibióticos. No se realizó un metanálisis debido a la falta de datos relevantes. De los cinco ensayos incluidos, un ensayo comparó cefazolina más amikacina con vancomicina más amikacina; un ensayo comparó ticarcilina más ácido clavulánico con flucloxacilina más gentamicina; un ensayo comparó cloxacilina más amikacina con cefotaxima más gentamicina; un ensayo comparó meropenem con atención estándar (ampicilina más gentamicina o cefotaxima más gentamicina); y un ensayo comparó vancomicina más gentamicina con vancomicina más aztreonam. Ninguna de las cinco comparaciones encontró evidencia de una diferencia al evaluar la mortalidad por todas las causas, los eventos adversos graves, el apoyo circulatorio, la nefrotoxicidad, el deterioro del desarrollo neurológico o la enterocolitis necrosante; sin embargo, ninguno de los ensayos se acercó a un tamaño de información que pudiera contribuir significativamente a la evidencia de los beneficios y los riesgos comparativos de cualquier régimen antibiótico en particular. Ninguno de los ensayos evaluó la asistencia respiratoria o la ototoxicidad. Los efectos beneficiosos y perjudiciales de los diferentes regímenes de antibióticos aún no están claros debido a la falta de ensayos con un poder estadístico adecuado y al alto riesgo de errores sistemáticos.
Conclusiones de los autores: La evidencia actual no es suficiente para apoyar que un régimen de antibióticos sea superior a otro. Se justifica la realización de ECA con bajo riesgo de sesgo que evalúen diferentes regímenes antibióticos en la sepsis neonatal de inicio tardío.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
The project received no funding.
SKK: none.
SS: none.
CN: none.
MG: none.
GG: none.
ULT: none.
JCJ: none.
Figures
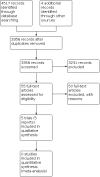
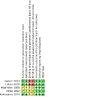















Update of
References
References to studies included in this review
Ceriani 2014 {published data only}
-
- Ceriani Cernadas JM, Fernandez Jonusas S, Marquez M, Garsd A, Mariani G. Clinical outcome of neonates with nosocomial suspected sepsis treated with cefazolin or vancomycin: a non-inferiority, randomized, controlled trial. Archivos Argentinos de Pediatría 2014;112(4):308-14. [DOI: 10.5546/aap.2014.308] [PMID: ] - DOI - PubMed
Lutsar 2020 {published data only}
-
- Lutsar I, Chazallon C, Trafojer U, Vincent MC, Cinzia A, Chiara B, et al, NeoMero Consortium. Meropenem vs standard of care for treatment of neonatal late onset sepsis (NeoMero1): a randomised controlled trial. Plos One 2020;15(3):e0229380. [DOI: 10.1371/journal.pone.0229380] [PMID: ] - DOI - PMC - PubMed
Miall‐Allen 1988 {published data only}
-
- Miall-Allen VM, Whitelaw AG, Darrell JH. Ticarcillin plus clavulanic acid (Timentin) compared with standard antibiotic regimes in the treatment of early and late neonatal infections. British Journal of Clinical Practice 1988;42(7):273-9. [PMID: ] - PubMed
Millar 1992 {published data only}
References to studies excluded from this review
Adelman 1987a {published data only}
Adelman 1987b {published data only}
Alinejad 2018 {published data only}
-
- Alinejad S, Yousefichaijan P, Rezagholizamenjany M, Rafie Y, Kahbazi M, Arjmand A. Nephrotoxic effect of gentamicin and amikacin in neonates with Infection. Nephro-Urology Monthly 2018;10(2):e58580. [DOI: 10.5812/numonthly.58580] - DOI
Aronoff 1984 {published data only}
Auriti 2005 {published data only}
Baqui 2013 {published data only}
-
- Baqui AH, Saha SK, Ahmed A, Shahidullah M, Quasem I, Roth DE, et al. Safety and efficacy of simplified antibiotic regimens for outpatient treatment of serious infection in neonates and young infants 0-59 days of age in Bangladesh: design of a randomized controlled trial. Pediatric Infectious Disease Journal 2013;32(Suppl 1):S12-8. [DOI: 10.1097/INF.0b013e31829ff790] [PMID: ] - DOI - PMC - PubMed
Bassetti 1991 {published data only}
-
- Bassetti D, Cruciani M, Solbiati M, Rubini F, Gandola L, Valenti G, et al. Comparative efficacy of ceftriaxone versus ceftazidime in the treatment of nosocomial lower respiratory tract infections. Chemotherapy 1991;37(5):371-5. [DOI: 10.1159/000238881] [PMID: ] - PubMed
Begue 1998 {published data only}
-
- Begue P, Astruc J, Francois P, Floret D. Comparison of ceftriaxone and cefotaxime in severe pediatric bacterial infections: a multicentrique study [Evaluation de la ceftriaxone et du cefotaxime dans l'infection bacterienne severe en pediatrie: etude multicentrique]. Medecine et Maladies Infectieuses 1998;28(4):300-6. [DOI: 10.1016/S0399-077X(98)80054-1] - DOI
Chartrand 1984 {published data only}
Chowdhary 2006 {published data only}
Collins 1998 {published data only}
-
- Collins MD, Dajani AS, Kim KS, King DR, Kaplan SL, Azimi PH, et al. Comparison of ampicillin/sulbactam plus aminoglycoside vs. ampicillin plus clindamycin plus aminoglycoside in the treatment of intraabdominal infections in children. The Multicenter Group. Pediatric Infectious Disease Journal 1998;17(3 Suppl):S15-8; discussion S20-1. [DOI: 10.1097/00006454-199803001-00005] [PMID: ] - DOI - PubMed
De Louvois 1992 {published data only}
-
- De Louvois J, Dagan R, Tessin I. A comparison of ceftazidime and aminoglycoside based regimens as empirical treatment in 1316 cases of suspected sepsis in the newborn. European Society for Paediatric Infectious Diseases – Neonatal Sepsis Study Group. European Journal of Pediatrics 1992;151(12):876-84. [DOI: 10.1007/BF01954122] [PMID: ] - DOI - PubMed
Deville 2003 {published data only}
-
- Deville JG, Adler S, Azimi PH, Jantausch BA, Morfin MR, Beltran S, et al. Linezolid versus vancomycin in the treatment of known or suspected resistant Gram-positive infections in neonates. Pediatric Infectious Disease Journal 2003;22(9 Suppl):S158-63. [DOI: 10.1097/01.inf.0000086955.93702.c7] [PMID: ] - DOI - PubMed
Ebrahim 1969 {published data only}
-
- Ebrahim GJ. Sepsis of the new-born (a therapeutic trial with gentamicin). East African Medical Journal 1969;46(1):30-3. [PMID: ] - PubMed
Faix 1988 {published data only}
Feigin 1976 {published data only}
-
- Feigin RD, Stechenberg BW, Chang MJ, Dunkle LM, Wong ML, Palkes H, et al. Prospective evaluation of treatment of Hemophilus influenzae meningitis. Journal of Pediatrics 1976;88(4 Pt 1):542-8. - PubMed
Fogel 1983 {published data only}
-
- Fogel D, Farfel L, Miskin A, Mogilner BM. Comparison between the combination of azlocillin-gentamicin and ampicillin-gentamicin in the treatment of a nursery population. Israel Journal of Medical Sciences 1983;19(11):1009-15. [PMID: ] - PubMed
Gathwala 2010 {published data only}
Gokalp 1991 {published data only}
-
- Gokalp AS, Oguz A, Gultekin A, Icagasioglu D. Neonatal sepsis in Turkey: the comparison between penicillin plus aminoglycoside and ampicillin plus third-generation cephalosporin chemotherapies. Materia Medica Polona. Polish Journal of Medicine and Pharmacy 1991;23(3):226-28. [DOI: 10.1093/tropej/36.4.200] [PMID: ] - DOI - PubMed
Haffejee 1984 {published data only}
Hall 1988 {published data only}
-
- Hall MA, Ducker DA, Lowes JA, McMichael J, Clarke P, Rowe D, et al. A randomised prospective comparison of cefotaxime versus netilmicin/penicillin for treatment of suspected neonatal sepsis. Drugs 1988;35(Suppl 2):169-77. - PubMed
Hammerberg 1989 {published data only}
-
- Hammerberg O, Kurnitzki C, Watts J, Rosenbloom D. Randomized trial using piperacillin versus ampicillin and amikacin for treatment of premature neonates with risk factors for sepsis. European Journal of Clinical Microbiology & Infectious Diseases 1989;8(3):241-4. [DOI: 10.1007/BF01965268] [PMID: ] - DOI - PubMed
Hansen 1980 {published data only}
Jantausch 2003 {published data only}
-
- Jantausch BA, Deville J, Adler S, Morfin MR, Lopez P, Edge-Padbury B, et al. Linezolid for the treatment of children with bacteremia or nosocomial pneumonia caused by resistant Gram-positive bacterial pathogens. Pediatric Infectious Disease Journal 2003;22(9 Suppl):S164-71. [DOI: 10.1097/01.inf.0000086956.45566.55] [PMID: ] - DOI - PubMed
Kaplan 2003 {published data only}
-
- Kaplan SL, Deville JG, Yogev R, Morfin MR, Wu E, Adler S, et al, Linezolid Pediatric Study Group. Linezolid versus vancomycin for treatment of resistant Gram-positive infections in children. Pediatric Infectious Disease Journal 2003;22(8):677-86. [DOI: 10.1097/01.inf.0000078160.29072.42] [PMID: ] - DOI - PubMed
Langhendries 1993 {published data only}
-
- Langhendries JP, Battisti O, Bertrand JM, François A, Darimont J, Tulkens PM, et al. Once daily administration of amikacin. Adaptation to neonatology for infants less than 3 days of postnatal age [Administration en dose unique journalière de l'amikacine. Adaptation à la néonatologie pour des enfants traités avant le 3ème jour d'âge postnatal]. Médecine et Maladies Infectieuses 1993;23(7):44-54. [DOI: 10.1016/S0399-077X(05)80984-9] - DOI
Lee 2005 {published data only}
-
- Lee SJ, Park EA. Efficacy and safety of amoxicillin-sulbactam and ampicillin-sulbactam in full term neonates. Journal of the Korean Society of Neonatology 2005;12(1):17-24.
Lönnerholm 1982 {published data only}
Marks 1978 {published data only}
McCracken 1976 {published data only}
Metsvaht 2010 {published data only}
-
- Metsvaht T, Ilmoja ML, Parm U, Maipuu L, Merila M, Lutsar I. Comparison of ampicillin plus gentamicin vs penicillin plus gentamicin in empiric treatment of neonates at risk of early onset sepsis. Acta Paediatrica, International Journal of Paediatrics 2010;99(5):665-72. [DOI: 10.1111/j.1651-2227.2010.01687.x] [PMID: ] - DOI - PubMed
-
- Metsvaht T, Ilmoja ML, Parm U, Merila M, Maipuu L, Muursepp P, et al. Ampicillin versus penicillin in the empiric therapy of extremely low-birthweight neonates at risk of early onset sepsis. Pediatrics International 2011;53(6):873-80. [DOI: 10.1111/j.1442-200X.2011.03468.x] [PMID: 21895866] - DOI - PubMed
-
- Parm U, Metsvaht T, Sepp E, Ilmoja ML, Pisarev H, Pauskar M, et al. Impact of empiric antibiotic regimen on bowel colonization in neonates with suspected early onset sepsis. European Journal of Clinical Microbiology & Infectious Diseases 2010;29(7):807-16. [DOI: 10.1007/s10096-010-0931-1] [PMID: ] - DOI - PubMed
Mir 2017 {published data only}
-
- Mir F, Nisar I, Tikmani SS, Baloch B, Shakoor S, Jehan F, et al. Simplified antibiotic regimens for treatment of clinical severe infection in the outpatient setting when referral is not possible for young infants in Pakistan (Simplified Antibiotic Therapy Trial [SATT]): a randomised, open-label, equivalence trial. Lancet. Global Health 2017;5(2):e177-85. [DOI: 10.1016/S2214-109X(16)30335-7] [PMID: ] - DOI - PMC - PubMed
Molyneux 2017 {published data only}
-
- Molyneux EM, Dube Q, Banda FM, Chiume M, Singini I, Mallewa M, et al. The treatment of possible severe infection in infants: an open randomized safety trial of parenteral benzylpenicillin and gentamicin versus ceftriaxone in Infants <60 days of age in Malawi. Pediatric Infectious Disease Journal 2017;36(12):e328-33. [DOI: 10.1097/INF.0000000000001576] [PMID: ] - DOI - PMC - PubMed
Mulubwa 2020 {published data only}
Odio 1987 {published data only}
Odio 1995 {published data only}
Oral 1998 {published data only}
Rohatgi 2017 {published data only}
Snelling 1983 {published data only}
Taheri 2011 {published data only}
-
- Taheri PA, Eslamieh H, Salamati P. Is ceftizoxime an appropriate surrogate for amikacin in neonatal sepsis treatment? A randomized clinical trial. Acta Medica Iranica 2011;49(8):499-503. [PMID: ] - PubMed
Tessin 1988 {published data only}
-
- Tessin I, Thiringer K, Trollfors B, Brorson JE. Comparison of serum concentrations of ceftazidime and tobramycin in newborn infants. European Journal of Pediatrics 1988;147(4):405-7. [DOI: 10.1007/BF00496420] [PMID: ] - PubMed
Tessin 1989 {published data only}
Tewari 2014 {published data only}
Tshefu 2015a {published data only}
-
- African Neonatal Sepsis Trial (AFRINEST) group, Tshefu A, Lokangaka A, Ngaima S, Engmann C, Esamai F, Gisore P, et al. Simplified antibiotic regimens compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with clinical signs of possible serious bacterial infection when referral is not possible: a randomised, open-label, equivalence trial. Lancet 2015;385(9979):1767-76. [DOI: 10.1016/S0140-6736(14)62284-4] [PMID: ] - DOI - PubMed
Tshefu 2015b {published data only}
-
- African Neonatal Sepsis Trial (AFRINEST) group, Tshefu A, Lokangaka A, Ngaima S, Engmann C, Esamai F, Gisore P, et al. Oral amoxicillin compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with fast breathing when referral is not possible: a randomised, open-label, equivalence trial. Lancet 2015;385(9979):1758-66. [DOI: 10.1016/S0140-6736(14)62285-6] [PMID: ] - DOI - PubMed
Umaña 1990 {published data only}
Viganò 1995 {published data only}
-
- Viganò A, Principi N. A randomised comparison of isepamicin and amikacin in the treatment of bacterial infections in paediatric patients. Journal of Chemotherapy 1995;7(Suppl 2):95-101. [PMID: ] - PubMed
Wells 1984 {published data only}
Wiese 1988 {published data only}
Zaidi 2013 {published data only}
-
- Zaidi AK, Tikmani SS, Sultana S, Baloch B, Kazi M, Rehman H, et al. Simplified antibiotic regimens for the management of clinically diagnosed severe infections in newborns and young infants in first-level facilities in Karachi, Pakistan: study design for an outpatient randomized controlled equivalence trial. Pediatric Infectious Disease Journal 2013;32(Suppl 1):S19-25. [DOI: 10.1097/INF.0b013e31829ff7aa] [PMID: ] - DOI - PMC - PubMed
Additional references
Allan 1985
Bakhuizen 2014
Barbateskovic 2021
Bedford Russell 2015
Bell 1978
Benjamin 2006
-
- Benjamin DK Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, et al, National Institute of Child Health and Human Development Neonatal Research Network. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 2006;117(1):84-92. [DOI: 10.1542/peds.2004-2292] [PMID: ] - DOI - PubMed
Bérdy 2005
Bhutta 1996
Bizzarro 2005
Bizzarro 2008
Bizzarro 2015
Boghossian 2013
-
- Boghossian NS, Page GP, Bell EF, Stoll BJ, Murray JC, Cotten CM, et al, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Late-onset sepsis in very low birth weight infants from singleton and multiple-gestation births. Journal of Pediatrics 2013;162(6):1120-4, 1124.e1. [DOI: 10.1016/j.jpeds.2012.11.089] [PMID: ] - DOI - PMC - PubMed
Breurec 2016
Brok 2008
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive – trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. International Journal of Epidemiology 2009;38(1):287-98. [DOI: 10.1093/ije/dyn188] [PMID: ] - DOI - PubMed
Camacho‐Gonzalez 2013
Cantey 2015
Clark 2006a
-
- Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics 2006;117(1):67-74. [DOI: 10.1542/peds.2005-0179] [PMID: ] - DOI - PubMed
Clark 2006b
Cohen 1992
Cordero 2003
Cortese 2016
Cotten 2006
-
- Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK Jr, National Institute for Child Health and Human Development Neonatal Research Network. The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics 2006;118(2):717-22. [DOI: 10.1542/peds.2005-2677] [PMID: ] - DOI - PubMed
Cotten 2009
-
- Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sánchez PJ, et al, NICHD Neonatal Research Network. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009;123(1):58-66. [DOI: 10.1542/peds.2007-3423] [PMID: ] - DOI - PMC - PubMed
Craft 2000
Dagnew 2013
-
- Dagnew M, Yismaw G, Gizachew M, Gadisa A, Abebe T, Tadesse T, et al. Bacterial profile and antimicrobial susceptibility pattern in septicemia suspected patients attending Gondar University Hospital, Northwest Ethiopia. BMC Research Notes 2013;6:283. [DOI: 10.1186/1756-0500-6-283] [PMID: ] - DOI - PMC - PubMed
Demets 1987
DerSimonian 1986
Dong 2015
Farber 1983
Fernando 2008
Filioti 2007
-
- Filioti J, Spiroglou K, Roilides E. Invasive candidiasis in pediatric intensive care patients: epidemiology, risk factors, management, and outcome. Intensive Care Medicine 2007;33(7):1272-83. [DOI: 10.1007/s00134-007-0672-5] [PMID: ] - PubMed
Fisher 1922
-
- Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society 1922;85(1):87-94. [DOI: 10.2307/2340521] - DOI
Foster 2006
Gillies 2015
-
- Gillies M, Ranakusuma A, Hoffmann T, Thorning S, McGuire T, Glasziou P, et al. Common harms from amoxicillin: a systematic review and meta-analysis of randomized placebo-controlled trials for any indication. Canadian Medical Association Journal 2015;187(1):E21-31. [DOI: 10.1503/cmaj.140848] [PMID: ] - DOI - PMC - PubMed
Golan 2011
-
- Golan DE, Tashlian AH, Armstrong EJ, Armstrong AW. Principles of Pharmacology. 3rd edition. Philadelphia (PA): Lippincott Williams & Wilkins, 2011.
Goldstein 2005
Gordon 2005
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 13 December 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hailemeskel 1999
-
- Hailemeskel B, Namanny M, Wutoh A. Frequency of nephrotoxicity with vancomycin and aminoglycoside antibiotic therapy. Hospital Pharmacy 1999;34(12):1417-20. [DOI: 10.1177/194512539903401209] - DOI
Hammoud 2012
Higgins 2002
Higgins 2003
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2019
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6. - PMC - PubMed
Horbar 2017
-
- Horbar JD, Edwards EM, Greenberg LT, Morrow KA, Soll RF, Buus-Frank ME, et al. Variation in performance of neonatal intensive care units in the United States. JAMA Pediatrics 2017;171(3):e164396. - PubMed
Huth 2011
Huynh 2016
Ibrahim 2000
ICH‐GCP 2015
-
- International Conference on Harmonisation-Good Clinical Practice. ICH harmonised guideline: integrated addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2) ICH Consensus Guideline (ICH-GCP), 2015. ichgcp.net (accessed prior to 19 April 2021).
Isaacs 1996
-
- Isaacs D, Barfield C, Clothier T, Darlow B, Diplock R, Ehrlich J, et al. Late-onset infections of infants in neonatal units. Journal of Paediatrics and Child Health 1996;32(2):158-61. [ 10.1111/j.1440-1754.1996.tb00914.x] [PMID: ] - PubMed
Isaacs 1999
-
- Isaacs D, Royle JA. Intrapartum antibiotics and early onset neonatal sepsis caused by group B Streptococcus and by other organisms in Australia. Australasian Study Group for Neonatal Infections. Pediatric Infectious Disease Journal 1999;18(6):524-8. [DOI: 10.1097/00006454-199906000-00009] [PMID: ] - DOI - PubMed
Isaacs 2003
-
- Isaacs D, Australasian Study Group for Neonatal Infections. A ten year, multicentre study of coagulase negative staphylococcal infections in Australasian neonatal units. Archives of Disease in Childhood. Fetal and Neonatal Edition 2003;88(2):F89-93. [DOI: 10.1136/fn.88.2.f89] [PMID: ] - DOI - PMC - PubMed
Jackson 1971
Jakobsen 2014
Kabwe 2016
Kan 2016
Katzung 2009
-
- Katzung BG, Masters SB, Trevor AJ. Basic and Clinical Pharmacology. 11th edition. New York (NY): McGraw-Hill Medical Publishing Division, 2009.
Khatua 1986
Klinger 2010
Kumar 2016
Kunin 1990
Kuppala 2011
Lawn 2005
Leal 2012
-
- Leal YA, Álvarez-Nemegyei J, Velázquez JR, Rosado-Quiab U, Diego-Rodríguez N, Paz-Baeza E, et al. Risk factors and prognosis for neonatal sepsis in southeastern Mexico: analysis of a four-year historic cohort follow-up. BMC Pregnancy and Childbirth 2012;12:48. [DOI: 10.1186/1471-2393-12-48] [PMID: ] - DOI - PMC - PubMed
Leibovici 1998
Leibovici 2016
Liu 2012
-
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al, Child Health Epidemiology Reference Group of WHO and UNICEF . Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379(9832):2151-61; Erratum in: Lancet 2012;380(9850):1308. [DOI: 10.1016/S0140-6736(12)60560-1] [PMID: ] - DOI - PubMed
Luck 2003
Manan 2016
Marchant 2013
Mattie 1989
-
- Mattie H, Craig WA, Pechère JC. Determinants of efficacy and toxicity of aminoglycosides. Journal of Antimicrobial Chemotherapy 1989;24(3):281-93. [DOI: 10.1093/jac/24.3.281] [PMID: ] - PubMed
May 2005
-
- May M, Daley AJ, Donath S, Isaacs D, Australasian Study Group for Neonatal Infections. Early onset neonatal meningitis in Australia and New Zealand, 1992-2002. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(4):F324-7. [DOI: 10.1136/adc.2004.066134] [PMID: 15878934 ] - DOI - PMC - PubMed
McGlone 2008
McGovern 2020
-
- McGovern M, Giannoni E, Kuester H, Turner MA, den Hoogen A, Bliss JM, et al. Challenges in developing a consensus definition of neonatal sepsis. Pediatric Research 2020;88(1):14-26. - PubMed
McGowan 1994
McWilliam 2017
Milatovic 1987
Mingeot‐Leclercq 1999
Moher 2009
Morris 2016
-
- Morris JM, Roberts CL, Bowen JR, Patterson JA, Bond DM, Algert CS, et al, PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet 2016;387(10017):444-52. [DOI: 10.1016/S0140-6736(15)00724-2] [PMID: ] - DOI - PubMed
Mrvos 2013
Muller‐Pebody 2011
Murray 1994
Musiime 2015
Oeser 2013
Papile 1978
Paul 2010
Review Manager 2020 [Computer program]
-
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rogosch 2012
Rubin 2002
Rybak 2009
-
- Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC Jr, Craig WA, Billeter M, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2009;29(11):1275-9. [DOI: 10.1592/phco.29.11.1275] [PMID: ] - DOI - PubMed
Sáez‐Llorens 2000
-
- Sáez-Llorens X, Castrejón de Wong MM, Castaño E, De Suman O, De Morös D, De Atencio I. Impact of an antibiotic restriction policy on hospital expenditures and bacterial susceptibilities: a lesson from a pediatric institution in a developing country. Pediatric Infectious Disease Journal 2000;19(3):200-6. [DOI: 10.1097/00006454-200003000-00005] [PMID: ] - DOI - PubMed
Schlapbach 2011
-
- Schlapbach LJ, Aebischer M, Adams M, Natalucci G, Bonhoeffer J, Latzin P, et al, Swiss Neonatal Network and Follow-Up Group. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics 2011;128(2):e348-57. [DOI: 10.1542/peds.2010-3338] [PMID: ] - DOI - PubMed
Schuchat 2000
Schultze 1971
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Seale 2015
Selimoglu 2007
Shah 2014
Shane 2013
Shane 2014
Sharma 2007
-
- Sharma R, Tepas JJ 3rd, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. Journal of Pediatric Surgery 2007;42(3):454-61. [DOI: 10.1016/j.jpedsurg.2006.10.038] [PMID: ] - DOI - PubMed
Singer 2016
Sorrell 1985
Spiliopoulou 2012
-
- Spiliopoulou A, Dimitriou G, Jelastopulu E, Giannakopoulos I, Anastassiou ED, Christofidou M. Neonatal intensive care unit candidemia: epidemiology, risk factors, outcome, and critical review of published case series. Mycopathologia 2012;173(4):219-28. [DOI: 10.1007/s11046-011-9498-3] [PMID: ] - DOI - PubMed
Stockmann 2014
Stoll 1996
-
- Stoll BJ, Gordon T, Korones SB, Shankaran S, Tyson JE, Bauer CR, et al. Late-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. Journal of Pediatrics 1996;129(1):63-71. [DOI: 10.1016/s0022-3476(96)70191-9] [PMID: ] - DOI - PubMed
Stoll 2002
Stoll 2003
Stoll 2005
-
- Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, et al, National Institute of Child Health and Human Development. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of Gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatric Infectious Disease Journal 2005;24(7):635-9. [DOI: 10.1097/01.inf.0000168749.82105.64] [PMID: ] - DOI - PubMed
Stoll 2010
-
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126(3):443-56. [DOI: 10.1542/peds.2009-2959] [PMID: ] - DOI - PMC - PubMed
Stoll 2011
-
- Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Meurs KP, et al, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics 2011;127(5):817-26. [DOI: 10.1542/peds.2010-2217] [PMID: ] - DOI - PMC - PubMed
Stoll 2015
Tallur 2000
Tamma 2012
Tessin 1990
Thorlund 2009
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 7 January 2015).
Tripathi 2012
Tröger 2014
-
- Tröger B, Göpel W, Faust K, Müller T, Jorch G, Felderhoff-Müser U, et al, German Neonatal Network. Risk for late-onset blood-culture proven sepsis in very-low-birth weight infants born small for gestational age: a large multicenter study from the German Neonatal Network. Pediatric Infectious Disease Journal 2014;33(3):238-43. [DOI: 10.1097/INF.0000000000000031] [PMID: ] - DOI - PubMed
TSA 2011
-
- Copenhagen Trial Unit. TSA – Trial Sequential Analysis, 2011. ctu.dk/tsa/ (accessed 7 January 2015).
Tsai 2014
Vergnano 2005
Vergnano 2011
Vesikari 1985
Volpe 2008
-
- Volpe JJ. Intracranial hemorrhage: germinal matrix intraventricular hemorrhage. In: Neurology of the Newborn. 5th edition. Philadelphia (PA): Saunders Elsevier, 2008:517-88.
Waksman 1947
Walker 2011
Wargo 2014
Weinstein 2003
Weston 2011
Wetterslev 2008
Wetterslev 2009
Wetterslev 2017
WHO 1999
WHO 2013
-
- World Health Organization. Pocket book of hospital care for children: second edition. Guidelines for the management of common childhood illnesses. apps.who.int/iris/bitstream/10665/81170/1/9789241548373_eng.pdf?ua=1 (accessed 16 February 2018).
World Bank 2017
-
- World Bank. World Bank country and lending groups. datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-coun... (accessed 9 December 2019).
Wynn 2014
Wynn 2016
Ygberg 2012
Zaidi 2005
Zaidi 2009
Zea‐Vera 2015
Zemlin 2007
-
- Zemlin M, Hoersch G, Zemlin C, Pohl-Schickinger A, Hummel M, Berek C, et al. The postnatal maturation of the immunoglobulin heavy chain IgG repertoire in human preterm neonates is slower than in term neonates. Journal of Immunology 2007;178(2):1180-8. [DOI: 10.4049/jimmunol.178.2.1180] [PMID: ] - DOI - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

