Safety, Tolerability, and Pharmacokinetics of FAAH Inhibitor BIA 10-2474: A Double-Blind, Randomized, Placebo-Controlled Study in Healthy Volunteers
- PMID: 33998672
- PMCID: PMC9292215
- DOI: 10.1002/cpt.2290
Safety, Tolerability, and Pharmacokinetics of FAAH Inhibitor BIA 10-2474: A Double-Blind, Randomized, Placebo-Controlled Study in Healthy Volunteers
Abstract
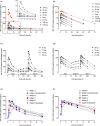
This study evaluated the safety, tolerability, pharmacokinetics, and pharmacodynamics of BIA 10-2474, a fatty acid amide hydrolase (FAAH) inhibitor, after first administration to healthy male and female participants. Participants (n = 116) were recruited into this phase I, double-blind, randomized, placebo-controlled, single ascending dose and multiple ascending dose (10-day) study. The primary outcome was the safety and tolerability of BIA 10-2474. Secondary outcomes were pharmacokinetics of BIA 10-2474 and pharmacodynamics, considering plasma concentrations of anandamide and three other fatty acid amides (FAAs) and leukocyte FAAH activity. Single oral doses of 0.25-100 mg and repeated oral doses of 2.5-50 mg were evaluated. BIA 10-2474 was well tolerated up to 100 mg as a single dose and up to 20 mg once daily for 10 days. In the cohort receiving repeated administrations of 50 mg, there were central nervous system adverse events in five of six participants, one with fatal outcome, which led to early termination of the study. BIA 10-2474 showed a linear relationship between dose and area under plasma concentration-time curve (AUC) across the entire dose range and reached steady state within 5-6 days of administration, with an accumulation ratio, based on AUC0-24h , of <2 on Day 10. BIA 10-2474 was rapidly absorbed with a mean terminal elimination half-life of 8-10 hours (Day 10). BIA 10-2474 caused reversible, dose-related increases in plasma FAAs. In conclusion, we propose that these data, as well as the additional data generated since the clinical trial was stopped, do not provide a complete mechanistic explanation for the tragic fatality.
© 2021 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
A.F., P.M., P.P., and A.W.H. received consulting fees from BIAL ‐ Portela & Cª, S.A. J.‐F.R., A.S., H.G., and P.S.‐d.‐S. were employees of BIAL at the time of the study, which was interrupted because of the occurrence of adverse reactions and is currently the subject of pending legal proceedings in France.
Figures

Comment in
-
De-risking Clinical Trials: The BIAL Phase I Trial in Foresight.Clin Pharmacol Ther. 2022 Feb;111(2):362-365. doi: 10.1002/cpt.2498. Epub 2021 Dec 28. Clin Pharmacol Ther. 2022. PMID: 34962650 Free PMC article. No abstract available.
-
BIA 10-2474: Some Lessons are Clear but Important Questions Remain Unanswered.Clin Pharmacol Ther. 2022 Feb;111(2):343-345. doi: 10.1002/cpt.2495. Clin Pharmacol Ther. 2022. PMID: 35007339 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

