Effectiveness of erenumab and onabotulinumtoxinA on acute medication usage and health care resource utilization as migraine prevention in the United States
- PMID: 33998825
- PMCID: PMC10394219
- DOI: 10.18553/jmcp.2021.21060
Effectiveness of erenumab and onabotulinumtoxinA on acute medication usage and health care resource utilization as migraine prevention in the United States
Abstract
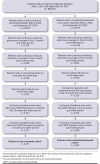
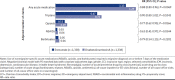
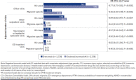
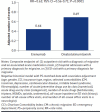
BACKGROUND: Migraine is a common neurological disease that can have a substantial impact on patients' lives and on society. Erenumab, a fully human monoclonal antibody that targets the calcitonin gene-related peptide receptor, was specifically developed for migraine prevention. The efficacy of erenumab has been established in several clinical trials; however, the real-world comparative effectiveness of erenumab has not been fully investigated. OBJECTIVE: To evaluate the real-world impact of erenumab and onabotulinumtoxinA on acute medication usage and health care resource utilization (HCRU) among patients with migraine in the United States. METHODS: This retrospective US claims analysis (Optum's deidentified Clinformatics Data Mart Database) evaluated patients aged at least 18 years diagnosed with migraine who initiated erenumab or onabotulinumtoxinA between May 1, 2018, and September 30, 2019 (index date: first erenumab/onabotulinumtoxinA claim). Cohorts were matched 1:1 using the propensity score (PS) method (greedy match with caliper = 0.1). Stratification was performed based on gender, chronic migraine without aura diagnosis, onabotulinumtoxinA use, and acute/preventive drug use. The impact of erenumab and onabotulinumtoxinA on acute medication usage and HCRU was assessed in the 6-month post-index period. An exploratory analysis assessed the impact of erenumab and onabotulinumtoxinA on a composite endpoint of: (1) outpatient visit with a migraine diagnosis and associated acute medication claim, (2) hospital admission with a primary migraine diagnosis, or (3) emergency department visit with a primary migraine diagnosis. PS-matched data were used for comparative analyses; logistic regression with covariate adjustment was used for dichotomous variables, and a negative binomial model was used for count variables, with odds ratios or rate ratios (RRs) and 95% CIs calculated. RESULTS: Following stratified PS matching, 1,338 patients were included in both cohorts. At 6 months, the adjusted average number of claims per person for any acute medication was significantly lower in the erenumab cohort (1.13 vs 1.29 in the onabotulinumtoxinA cohort; RR = 0.88; 95% CI = 0.80-0.96; P = 0.0069), although the difference in the number of claims for triptans and barbiturates was statistically nonsignificant. The adjusted average number of all-cause and migraine-specific visits per person to health care providers was generally lower in the erenumab cohort compared with the onabotulinumtoxinA cohort. Patients in the erenumab cohort had a significantly lower number of composite events (0.44 vs 0.69 in the onabotulinumtoxinA cohort; RR = 0.63; 95% CI = 0.56-0.71; P < 0.0001). Similarly, the adjusted proportion of patients with any of the 3 composite events was lower in the erenumab cohort (31.7% vs 44.3% in the onabotulinumtoxinA cohort; OR = 0.59; 95% CI = 0.49-0.70; P < 0.0001). CONCLUSIONS: In this retrospective claims analysis study, erenumab significantly reduced acute medication usage (opioids and nonsteroidal anti-inflammatory drugs; any acute medication when analyzed together) and HCRU to a greater extent than onabotulinumtoxinA. DISCLOSURES: This study was supported by Novartis Pharma AG. Novartis employees contributed to the study design, analysis of the data, and the decision to publish the results. Fang, Abdrabboh, Glassberg, Vo, and Ferraris are employed by Novartis. Zhou and Shen are employed by KMK Consulting, Inc., which received funding from Novartis to conduct the study. Tepper reports grants from Allergan, Amgen, ElectroCore, Eli Lilly, Lundbeck, Neurolief, Novartis, Satsuma, and Zosano, outside the submitted work; personal fees from Dartmouth-Hitchcock Medical Center, American Headache Society, Thomas Jefferson University, Aeon, Align Strategies, Allergan/AbbVie, Alphasights, Amgen, Aperture Venture Partners, Aralez Pharmaceuticals Canada, Axsome Therapeutics, Becker Pharmaceutical Consulting, BioDelivery Sciences International, Biohaven, ClearView Healthcare Partners, CoolTech, CRG, Currax, Decision Resources, DeepBench, DRG, Eli Lilly, Equinox, ExpertConnect, GLG, Guidepoint Global Healthcare Consultancy Group, Health Science Communications, HMP Communications, Impel, InteractiveForums, M3 Global Research, Magellan Rx Management, Medicxi, Navigant Consulting, Neurorelief, Nordic BioTech, Novartis, Pulmatrix, Reckner Healthcare, Relevale, SAI MedPartners, Satsuma, Slingshot Insights, Spherix Global Insights, Sudler and Hennessey, Synapse Medical Communications, System Analytic, Teva, Theranica, Thought Leader Select, Trinity Partners, XOC, Zosano, Krog and Partners, and Lundbeck, outside the submitted work; and CME honoraria from American Academy of Neurology, American Headache Society, Cleveland Clinic Foundation, Diamond Headache Clinic, Elsevier, Forefront Collaborative, Hamilton General Hospital, Ontario, Canada, Headache Cooperative of New England, Henry Ford Hospital, Detroit, Inova, Medical Learning Institute PeerView, Medical Education Speakers Network, Miller Medical Communications, North American Center for CME, Physicians' Education Resource, Rockpointe, ScientiaCME, WebMD/Medscape. The abstract and poster of these results were presented at The Migraine Trust Virtual Symposium (MTIS), October 3-9, 2020.
Conflict of interest statement
This study was supported by Novartis Pharma AG. Novartis employees contributed to the study design, analysis of the data, and the decision to publish the results. Fang, Abdrabboh, Glassberg, Vo, and Ferraris are employed by Novartis. Zhou and Shen are employed by KMK Consulting, Inc., which received funding from Novartis to conduct the study.
Tepper reports grants from Allergan, Amgen, ElectroCore, Eli Lilly, Lundbeck, Neurolief, Novartis, Satsuma, and Zosano, outside the submitted work; personal fees from Dartmouth-Hitchcock Medical Center, American Headache Society, Thomas Jefferson University, Aeon, Align Strategies, Allergan/AbbVie, Alphasights, Amgen, Aperture Venture Partners, Aralez Pharmaceuticals Canada, Axsome Therapeutics, Becker Pharmaceutical Consulting, BioDelivery Sciences International, Biohaven, ClearView Healthcare Partners, CoolTech, CRG, Currax, Decision Resources, DeepBench, DRG, Eli Lilly, Equinox, ExpertConnect, GLG, Guidepoint Global Healthcare Consultancy Group, Health Science Communications, HMP Communications, Impel, InteractiveForums, M3 Global Research, Magellan Rx Management, Medicxi, Navigant Consulting, Neurorelief, Nordic BioTech, Novartis, Pulmatrix, Reckner Healthcare, Relevale, SAI MedPartners, Satsuma, Slingshot Insights, Spherix Global Insights, Sudler and Hennessey, Synapse Medical Communications, System Analytic, Teva, Theranica, Thought Leader Select, Trinity Partners, XOC, Zosano, Krog and Partners, and Lundbeck, outside the submitted work; and CME honoraria from American Academy of Neurology, American Headache Society, Cleveland Clinic Foundation, Diamond Headache Clinic, Elsevier, Forefront Collaborative, Hamilton General Hospital, Ontario, Canada, Headache Cooperative of New England, Henry Ford Hospital, Detroit, Inova, Medical Learning Institute PeerView, Medical Education Speakers Network, Miller Medical Communications, North American Center for CME, Physicians’ Education Resource, Rockpointe, ScientiaCME, WebMD/Medscape.
The abstract and poster of these results were presented at The Migraine Trust Virtual Symposium (MTIS), October 3-9, 2020.
Figures
References
-
- Migraine Research Foundation. Migraine facts. Accessed December 14 2020. https://migraineresearchfoundation.org/about-migraine/migraine-facts/
-
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808. - PubMed
-
- Hawkins K, Wang S, Rupnow M. Direct cost burden among insured US employees with migraine. Headache. 2008;48(4):553-63. - PubMed
-
- Bonafede M, Sapra S, Shah N, Tepper S, Cappell K, Desai P. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache. 2018;58(5):700-14. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

