Cannabinoid-2 Agonism with AM2301 Mitigates Morphine-Induced Respiratory Depression
- PMID: 33998869
- PMCID: PMC8612410
- DOI: 10.1089/can.2020.0076
Cannabinoid-2 Agonism with AM2301 Mitigates Morphine-Induced Respiratory Depression
Abstract
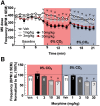
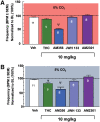
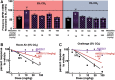
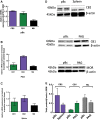
Introduction: An escalating number of fatalities resulting from accidental opioid overdoses typically attributed to respiratory depression continue to define the opioid epidemic. Opioid respiratory depression results from a decrease in reflexive inspiration within the preBötzinger complex in the brainstem. Objective: Cannabinoid receptor agonism is reported to enhance opioid analgesia, yet whether cannabinoids enhance or inhibit opioid-induced respiratory depression is unknown. Methods: Studies herein sought to define the roles of cannabinoid-1 receptor (CB1R) and cannabinoid-2 receptor (CB2R) on respiratory depression using selective agonists alone and in combination with morphine in male mice. Results: Using whole body plethysmography, the nonselective CB1R and CB2R agonist (Δ9-tetrahydrocannabinol) and the CB1R synthetic cannabinoid, AM356, induced respiratory depression, whereas the well-published selective CB2 agonist, JWH 133, and the novel CB2 agonist (AM2301) did not. Moreover, a selective CB2R agonist (AM2301) significantly attenuated morphine sulfate-induced respiratory depression. Conclusion: Notably, findings suggest that attenuation of opioid-induced respiratory depression relies on CB2R activation, supporting selective CB2R agonism as an opioid adjunct therapy.
Keywords: cannabinoid receptor 1; cannabinoid receptor 2; mu opioid receptor; opioid-induced respiratory depression; preBötzinger complex.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- NASEM. Pain management and the opioid epidemic: balancing societal and individual benefits and risks of prescription opioid use. The National Academies Press: Washington, DC, 2017. - PubMed
-
- Markos JR, Harris HM, Gul W, ElSohly MA, Sufka KJ. Effects of cannabidiol on morphine conditioned place preference in mice. Planta Medica. 2018;84:221–224. - PubMed
-
- Control CfD, Prevention. Understanding the epidemic. 2017;11. Available at https://www.cdc.gov/drugoverdose/epidemic/index.html Accessed October 15, 2020.
-
- Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112:226–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

