The Cannabinoid Receptor Agonist, WIN-55212-2, Suppresses the Activation of Proinflammatory Genes Induced by Interleukin 1 Beta in Human Astrocytes
- PMID: 33998879
- PMCID: PMC8864424
- DOI: 10.1089/can.2020.0128
The Cannabinoid Receptor Agonist, WIN-55212-2, Suppresses the Activation of Proinflammatory Genes Induced by Interleukin 1 Beta in Human Astrocytes
Abstract
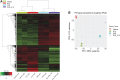
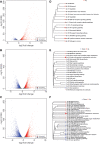
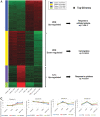
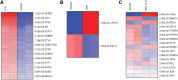
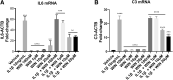
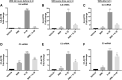
Background: Alterations of astrocyte function play a crucial role in neuroinflammatory diseases due to either the loss of their neuroprotective role or the gain of their toxic inflammatory properties. Accumulating evidence highlights that cannabinoids and cannabinoid receptor agonists, such as WIN55,212-2 (WIN), reduce inflammation in cellular and animal models. Thus, the endocannabinoid system has become an attractive target to attenuate chronic inflammation in neurodegenerative diseases. However, the mechanism of action of WIN in astrocytes remains poorly understood. Objective: We studied the immunosuppressive property of WIN by examining gene expression patterns that were modulated by WIN in reactive astrocytes. Materials and Methods: Transcriptomic analysis by RNA-seq was carried out using primary human astrocyte cultures stimulated by the proinflammatory cytokine interleukin 1 beta (IL1β) in the presence or absence of WIN. Real-time quantitative polymerase chain reaction analysis was conducted on selected transcripts to characterize the dose-response effects of WIN, and to test the effect of selective antagonists of cannabinoid receptor 1 (CB1) and peroxisome proliferator-activated receptors (PPAR). Results: Transcriptomic analysis showed that the IL1β-induced inflammatory response is robustly inhibited by WIN pretreatment. WIN treatment alone also induced substantial gene expression changes. Pathway analysis revealed that the anti-inflammatory properties of WIN were linked to the regulation of kinase pathways and gene targets of neuroprotective transcription factors, including PPAR and SMAD (mothers against decapentaplegic homolog). The inhibitory effect of WIN was dose-dependent, but it was not affected by selective antagonists of CB1 or PPAR. Conclusions: This study suggests that targeting the endocannabinoid system may be a promising strategy to disrupt inflammatory pathways in reactive astrocytes. The anti-inflammatory activity of WIN is independent of CB1, suggesting that alternative receptors mediate the effects of WIN. These results provide mechanistic insights into the anti-inflammatory activity of WIN and highlight that astrocytes are a potential therapeutic target to ameliorate neuroinflammation in the brain.
Keywords: immunosuppression; inflammation; neurobiology; synthetic cannabinoids.
Conflict of interest statement
No competing financial interests exist.
Figures
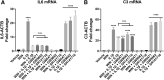
Similar articles
-
WIN 55,212-2, agonist of cannabinoid receptors, prevents amyloid β1-42 effects on astrocytes in primary culture.PLoS One. 2015 Apr 13;10(4):e0122843. doi: 10.1371/journal.pone.0122843. eCollection 2015. PLoS One. 2015. PMID: 25874692 Free PMC article.
-
WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-γ pathway.Neuropharmacology. 2012 Sep;63(4):653-66. doi: 10.1016/j.neuropharm.2012.05.013. Epub 2012 May 23. Neuropharmacology. 2012. PMID: 22634229
-
Inhibition of interleukin-1β-induced endothelial tissue factor expression by the synthetic cannabinoid WIN 55,212-2.Oncotarget. 2016 Sep 20;7(38):61438-61457. doi: 10.18632/oncotarget.11367. Oncotarget. 2016. PMID: 27556861 Free PMC article.
-
An update on PPAR activation by cannabinoids.Br J Pharmacol. 2016 Jun;173(12):1899-910. doi: 10.1111/bph.13497. Epub 2016 May 19. Br J Pharmacol. 2016. PMID: 27077495 Free PMC article. Review.
-
Is It Time to Test the Antiseizure Potential of Palmitoylethanolamide in Human Studies? A Systematic Review of Preclinical Evidence.Brain Sci. 2022 Jan 12;12(1):101. doi: 10.3390/brainsci12010101. Brain Sci. 2022. PMID: 35053844 Free PMC article. Review.
Cited by
-
Alterations in Brain Cannabinoid Receptor Levels Are Associated with HIV-Associated Neurocognitive Disorders in the ART Era: Implications for Therapeutic Strategies Targeting the Endocannabinoid System.Viruses. 2021 Aug 31;13(9):1742. doi: 10.3390/v13091742. Viruses. 2021. PMID: 34578323 Free PMC article.
-
Region-Specific Impact of Repeated Synthetic Cannabinoid Exposure and Withdrawal on Endocannabinoid Signaling, Gliosis, and Inflammatory Markers in the Prefrontal Cortex and Hippocampus.Biomolecules. 2025 Mar 14;15(3):417. doi: 10.3390/biom15030417. Biomolecules. 2025. PMID: 40149953 Free PMC article.
-
Astroglial CB1 Cannabinoid Receptors Mediate CP 55,940-Induced Conditioned Place Aversion Through Cyclooxygenase-2 Signaling in Mice.Front Cell Neurosci. 2021 Nov 23;15:772549. doi: 10.3389/fncel.2021.772549. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34887729 Free PMC article.
-
Astrocyte Activation is A Potential Mechanism Underlying Depressed Mood and Apathy in People with HIV.J Neurol Psychol. 2022;9(1):05. doi: 10.13188/2332-3469.1000048. Epub 2022 Dec 26. J Neurol Psychol. 2022. PMID: 37205974 Free PMC article.
-
Mechanisms underlying HIV-associated cognitive impairment and emerging therapies for its management.Nat Rev Neurol. 2023 Nov;19(11):668-687. doi: 10.1038/s41582-023-00879-y. Epub 2023 Oct 10. Nat Rev Neurol. 2023. PMID: 37816937 Free PMC article. Review.
References
-
- Colombo E, Farina C. Astrocytes: key regulators of neuroinflammation. Trends Immunol. 2016;37:608–620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

