A Phase 2 Randomized Controlled Trial of the Efficacy and Safety of Cannabidivarin as Add-on Therapy in Participants with Inadequately Controlled Focal Seizures
- PMID: 33998885
- PMCID: PMC8713263
- DOI: 10.1089/can.2020.0075
A Phase 2 Randomized Controlled Trial of the Efficacy and Safety of Cannabidivarin as Add-on Therapy in Participants with Inadequately Controlled Focal Seizures
Abstract
Objective: We assessed the efficacy, safety, and tolerability of cannabidivarin (CBDV) as add-on therapy in adults with inadequately controlled focal seizures. Materials and Methods: One hundred and sixty-two participants (CBDV n=81; placebo n=81) were enrolled. After a 4-week baseline, participants titrated from 400 to 800 mg CBDV twice daily (b.i.d.) (or placebo) over 2 weeks, followed by 6 weeks stable dosing (at 800 mg b.i.d.) and a 12-day taper period. The primary endpoint was the change from baseline in focal seizure frequency during the 8-week treatment period. Secondary endpoints included additional efficacy measures relating to seizures, physician- and participant-reported outcomes, change in the use of rescue medication, cognitive assessments, and safety. Results: Median baseline focal seizure frequencies were 17-18 per 28 days in both groups, and similar reductions in frequency were observed in the CBDV (40.5%) and placebo (37.7%) groups during the treatment period (treatment ratio [% reduction] CBDV/placebo: 0.95 [4.6]; confidence interval: 0.78-1.17 [-16.7 to 21.9]; p=0.648). There were no differences between the CBDV and placebo groups for any seizure subtype. There were no significant treatment differences between CBDV and placebo groups for any of the secondary efficacy outcome measures. Overall, 59 (72.8%) of participants in the CBDV group and 39 (48.1%) in the placebo group had ≥1 treatment-emergent adverse event (AE); the 3 most common were diarrhea, nausea, and somnolence. The incidence of serious AEs was low (3.7% in the CBDV group vs. 1.2% in the placebo group). There was little or no effect of CBDV on vital signs, physical examination, or electrocardiogram findings. Elevations in serum transaminases (alanine aminotransferase or aspartate aminotransferase) to levels >3×upper limit of normal occurred in three participants taking CBDV (two discontinued as a result) and one taking placebo; however, none met the criteria for potential Hy's Law cases. Conclusion: It is likely the 40.5% seizure reduction with CBDV represents an appropriate pharmacological response in this population with focal seizures. The placebo response was, however, high, which may reflect the participants' expectations of CBDV, and a treatment difference from placebo was not observed. CBDV was generally well tolerated. Clinical Trial Registration number: NCT02365610.
Keywords: GWP42006; antiepileptic drug; cannabinoid; epilepsy.
Conflict of interest statement
M.N., F.S., and A.S. are employed by GW Research Ltd and hold share options in GW Research Ltd. L.P. has received consultation fees from GL Pharma, Mylan, Sandoz, and Vipharm. He has also received educational grants and support from Vipharm, GP Pharma, and Mylan. He has accepted honoraria and guest lecture fees for symposia and other events sponsored by Astellas, Lundbeck, Teva, Eisai, and GL Pharma. He has served as principal investigator for >50 sponsor-initiated studies, and has been a national coordinator and key opinion leader for Astellas. He has served as an expert witness and consultant for lawsuits, providing deposition and testimony, including analysis of medical documents. J.W.S. received research grants from UCB and personal fees from UCB and Zogenix outside the submitted work. P.C. has served mainly as principal investigator in 66 industry-sponsored clinical trials in epilepsy and migraine. In seven trials, he was country coordinator. He has co-organized 26 annual conferences, named Psychosocial Aspects of Epilepsy sponsored by Pfizer, Glaxo, and Sanofi in the past and UCB Pharma, Teva, Apotex, Mylan, Glenmark, Accord, and Novartis currently. He has accepted speaking fees for lectures and webinars sponsored by UCB Pharma.
Figures
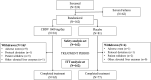
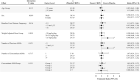
References
-
- Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am J Bot. 2004;91:966–975. - PubMed
-
- Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5:1131–1141. - PubMed
-
- Posner K, Brent D, Lucas C, et al. Columbia-Suicide Severity Rating Scale (C-SSRS). New York State Psychiatric Institute: New York, NY, 2008.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

