Protective Effects of Cannabidivarin and Cannabigerol on Cells of the Blood-Brain Barrier Under Ischemic Conditions
- PMID: 33998890
- PMCID: PMC8380798
- DOI: 10.1089/can.2020.0159
Protective Effects of Cannabidivarin and Cannabigerol on Cells of the Blood-Brain Barrier Under Ischemic Conditions
Abstract
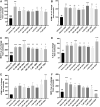
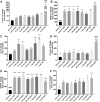
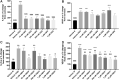
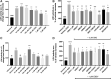
Background and Objectives: Preclinical studies have shown cannabidiol is protective in models of ischemic stroke. Based on results from our recent systematic review, we investigated the effects of two promising neuroprotective phytocannabinoids, cannabigerol (CBG) and cannabidivarin (CBDV), on cells of the blood-brain barrier (BBB), namely human brain microvascular endothelial cells (HBMECs), pericytes, and astrocytes. Experimental Approach: Cultures were subjected to oxygen-glucose deprivation (OGD) protocol to model ischemic stroke and cell culture medium was assessed for cytokines and adhesion molecules post-OGD. Astrocyte cell lysates were also analyzed for DNA damage markers. Antagonist studies were conducted where appropriate to study receptor mechanisms. Results: In astrocytes CBG and CBDV attenuated levels of interleukin-6 (IL-6) and lactate dehydrogenase (LDH), whereas CBDV (10 nM-10 μM) also decreased vascular endothelial growth factor (VEGF) secretion. CBDV (300 nM-10 μM) attenuated levels of monocyte chemoattractant protein (MCP)-1 in HBMECs. In astrocytes, CBG decreased levels of DNA damage proteins, including p53, whereas CBDV increased levels of DNA damage markers. Antagonists for CB1, CB2, PPAR-γ, PPAR-α, 5-HT1A, and TRPV1 had no effect on CBG (3 μM) or CBDV (1 μM)-mediated decreases in LDH in astrocytes. GPR55 and GPR18 were partially implicated in the effects of CBDV, but no molecular target was identified for CBG. Conclusions: We show that CBG and CBDV were protective against OG mediated injury in three different cells that constitute the BBB, modulating different hallmarks of ischemic stroke pathophysiology. These data enhance our understanding of the protective effects of CBG and CBDV and warrant further investigation into these compounds in ischemic stroke. Future studies should identify other possible neuroprotective effects of CBG and CBDV and their corresponding mechanisms of action.
Keywords: blood–brain barrier; cannabidivarin; cannabigerol; cannabinoids; ischemia; neuroprotection.
Conflict of interest statement
S.O.S. is director of CanPharmaConsulting, scientific advisor to pharmaceutical companies developing cannabinoid-based medicines. None of these companies were involved in this research.
Figures
References
-
- Abbott NJ, Patabendige AAK, Dolman DEM, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25 - PubMed
-
- Douglas DR, Luoma V, Reddy U. Acute management of ischaemic stroke. Anaesth Intensive Care Med. 2020;21:1–7
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

