A Multicriteria Decision Analysis Comparing Pharmacotherapy for Chronic Neuropathic Pain, Including Cannabinoids and Cannabis-Based Medical Products
- PMID: 33998895
- PMCID: PMC9418467
- DOI: 10.1089/can.2020.0129
A Multicriteria Decision Analysis Comparing Pharmacotherapy for Chronic Neuropathic Pain, Including Cannabinoids and Cannabis-Based Medical Products
Abstract
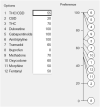
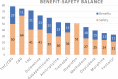
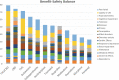
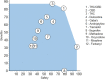
Background: Pharmacological management of chronic neuropathic pain (CNP) still represents a major clinical challenge. Collective harnessing of both the scientific evidence base and clinical experience (of clinicians and patients) can play a key role in informing treatment pathways and contribute to the debate on specific treatments (e.g., cannabinoids). A group of expert clinicians (pain specialists and psychiatrists), scientists, and patient representatives convened to assess the relative benefit-safety balance of 12 pharmacological treatments, including orally administered cannabinoids/cannabis-based medicinal products, for the treatment of CNP in adults. Methods: A decision conference provided the process of creating a multicriteria decision analysis (MCDA) model, in which the group collectively scored the drugs on 17 effect criteria relevant to benefits and safety and then weighted the criteria for their clinical relevance. Findings: Cannabis-based medicinal products consisting of tetrahydrocannabinol/cannabidiol (THC/CBD), in a 1:1 ratio, achieved the highest overall score, 79 (out of 100), followed by CBD dominant at 75, then THC dominant at 72. Duloxetine and the gabapentinoids scored in the 60s, amitriptyline, tramadol, and ibuprofen in the 50s, methadone and oxycodone in the 40s, and morphine and fentanyl in the 30s. Sensitivity analyses showed that even if the pain reduction and quality-of-life scores for THC/CBD and THC are halved, their benefit-safety balances remain better than those of the noncannabinoid drugs. Interpretation: The benefit-safety profiles for cannabinoids were higher than for other commonly used medications for CNP largely because they contribute more to quality of life and have a more favorable side effect profile. The results also reflect the shortcomings of alternative pharmacological treatments with respect to safety and mitigation of neuropathic pain symptoms. Further high-quality clinical trials and systematic comprehensive capture of clinical experience with cannabinoids is warranted. These results demonstrate once again the complexity and multimodal mechanisms underlying the clinical experience and impact of chronic pain.
Keywords: CBMP; MCDA; analgesics; cannabis-based medical products; multicriteria decision analysis; neuropathic pain.
Conflict of interest statement
M.P.B. is director of Maple Tree Consultants. D.P.F reports an Industry-Academia research grant from Alkermes, Inc., and Science Foundation Ireland outside of the submitted study. He also reports research grants in the area of cannabinoids or the endocannabinoid system from Shionogi Ltd. (Shionogi Science Programme), from B. Braun Ltd. jointly with Science Foundation Ireland, and from the Irish Research Council, CNPq Brazil, and EU INTERREG Programmes. T.H. is director of Clinic Horsted. J.M. is scientific advisor to Thedrug.store, a retailer of CBD products and natural supplements, and she also runs an online blog writing about cannabinoid science and general health and well-being. The blog does not generate an income and does not run advertisements. S.E.O'S. is scientific advisor for Artelo Biosciences, Dragonfly Biosciences, FSDPharma, Therapix Biosciences, and MJResults, and Science Lead of The Centre for Medicinal Cannabis. H.S. is on the scientific advisory board of Cellen Inc. D.J.N. is chair of the charity Drug Science. A.K.S. is head of research of the charity Drug Science. Drug Science receives an unrestricted educational grant from a consortium of medical cannabis companies. C.S. was clinical director of Drug Science's Project Twenty21. G.Z. is chief scientific officer and scientific board member at Cannaray Ltd., advisory board member and teacher at Masterclass Medicinal Cannabis, and scientific board member at Portugal Medical Cannabis (PTMC). All other authors declare no competing interests.
Figures



Similar articles
-
Intrathecal Actions of the Cannabis Constituents Δ(9)-Tetrahydrocannabinol and Cannabidiol in a Mouse Neuropathic Pain Model.Int J Mol Sci. 2022 Aug 3;23(15):8649. doi: 10.3390/ijms23158649. Int J Mol Sci. 2022. PMID: 35955774 Free PMC article.
-
Differences in prescribed medicinal cannabis use by cannabinoid product composition: Findings from the cannabis as medicine survey 2020 (CAMS-20) Australia-wide study.PLoS One. 2024 Feb 14;19(2):e0297092. doi: 10.1371/journal.pone.0297092. eCollection 2024. PLoS One. 2024. PMID: 38354169 Free PMC article.
-
Antinociceptive effects of minor cannabinoids, terpenes and flavonoids in Cannabis.Behav Pharmacol. 2022 Apr 1;33(2&3):130-157. doi: 10.1097/FBP.0000000000000627. Behav Pharmacol. 2022. PMID: 33709984 Review.
-
Knowledge of Tetrahydrocannabinol and Cannabidiol Levels Among Cannabis Consumers in the United States and Canada.Cannabis Cannabinoid Res. 2022 Jun;7(3):345-354. doi: 10.1089/can.2020.0092. Epub 2020 Oct 29. Cannabis Cannabinoid Res. 2022. PMID: 33998866 Free PMC article.
-
Current evidence of cannabinoid-based analgesia obtained in preclinical and human experimental settings.Eur J Pain. 2018 Mar;22(3):471-484. doi: 10.1002/ejp.1148. Epub 2017 Nov 21. Eur J Pain. 2018. PMID: 29160600 Review.
Cited by
-
Cys-loop receptors on cannabinoids: All high?Front Physiol. 2022 Nov 9;13:1044575. doi: 10.3389/fphys.2022.1044575. eCollection 2022. Front Physiol. 2022. PMID: 36439263 Free PMC article.
-
Phytocannabinoid Compositions from Cannabis Act Synergistically with PARP1 Inhibitor against Ovarian Cancer Cells In Vitro and Affect the Wnt Signaling Pathway.Molecules. 2022 Nov 3;27(21):7523. doi: 10.3390/molecules27217523. Molecules. 2022. PMID: 36364346 Free PMC article.
-
Overview: Chronic Pain and Cannabis-Based Medicines.Pharmacopsychiatry. 2024 May;57(3):152-159. doi: 10.1055/a-2231-6630. Epub 2024 Jan 10. Pharmacopsychiatry. 2024. PMID: 38198809 Free PMC article. Review.
-
[Cannabis- Summing it all up!].Anaesthesist. 2021 Jul;70(7):549-550. doi: 10.1007/s00101-021-01004-8. Epub 2021 Jul 7. Anaesthesist. 2021. PMID: 34232325 German. No abstract available.
-
Clinical Benefits and Safety of Medical Cannabis Products: A Narrative Review on Natural Extracts.Pain Ther. 2024 Oct;13(5):1063-1094. doi: 10.1007/s40122-024-00643-0. Epub 2024 Aug 3. Pain Ther. 2024. PMID: 39096481 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
