A Multicriteria Decision Analysis Comparing Pharmacotherapy for Chronic Neuropathic Pain, Including Cannabinoids and Cannabis-Based Medical Products
- PMID: 33998895
- PMCID: PMC9418467
- DOI: 10.1089/can.2020.0129
A Multicriteria Decision Analysis Comparing Pharmacotherapy for Chronic Neuropathic Pain, Including Cannabinoids and Cannabis-Based Medical Products
Abstract
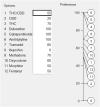
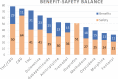
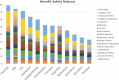
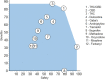
Background: Pharmacological management of chronic neuropathic pain (CNP) still represents a major clinical challenge. Collective harnessing of both the scientific evidence base and clinical experience (of clinicians and patients) can play a key role in informing treatment pathways and contribute to the debate on specific treatments (e.g., cannabinoids). A group of expert clinicians (pain specialists and psychiatrists), scientists, and patient representatives convened to assess the relative benefit-safety balance of 12 pharmacological treatments, including orally administered cannabinoids/cannabis-based medicinal products, for the treatment of CNP in adults. Methods: A decision conference provided the process of creating a multicriteria decision analysis (MCDA) model, in which the group collectively scored the drugs on 17 effect criteria relevant to benefits and safety and then weighted the criteria for their clinical relevance. Findings: Cannabis-based medicinal products consisting of tetrahydrocannabinol/cannabidiol (THC/CBD), in a 1:1 ratio, achieved the highest overall score, 79 (out of 100), followed by CBD dominant at 75, then THC dominant at 72. Duloxetine and the gabapentinoids scored in the 60s, amitriptyline, tramadol, and ibuprofen in the 50s, methadone and oxycodone in the 40s, and morphine and fentanyl in the 30s. Sensitivity analyses showed that even if the pain reduction and quality-of-life scores for THC/CBD and THC are halved, their benefit-safety balances remain better than those of the noncannabinoid drugs. Interpretation: The benefit-safety profiles for cannabinoids were higher than for other commonly used medications for CNP largely because they contribute more to quality of life and have a more favorable side effect profile. The results also reflect the shortcomings of alternative pharmacological treatments with respect to safety and mitigation of neuropathic pain symptoms. Further high-quality clinical trials and systematic comprehensive capture of clinical experience with cannabinoids is warranted. These results demonstrate once again the complexity and multimodal mechanisms underlying the clinical experience and impact of chronic pain.
Keywords: CBMP; MCDA; analgesics; cannabis-based medical products; multicriteria decision analysis; neuropathic pain.
Conflict of interest statement
M.P.B. is director of Maple Tree Consultants. D.P.F reports an Industry-Academia research grant from Alkermes, Inc., and Science Foundation Ireland outside of the submitted study. He also reports research grants in the area of cannabinoids or the endocannabinoid system from Shionogi Ltd. (Shionogi Science Programme), from B. Braun Ltd. jointly with Science Foundation Ireland, and from the Irish Research Council, CNPq Brazil, and EU INTERREG Programmes. T.H. is director of Clinic Horsted. J.M. is scientific advisor to Thedrug.store, a retailer of CBD products and natural supplements, and she also runs an online blog writing about cannabinoid science and general health and well-being. The blog does not generate an income and does not run advertisements. S.E.O'S. is scientific advisor for Artelo Biosciences, Dragonfly Biosciences, FSDPharma, Therapix Biosciences, and MJResults, and Science Lead of The Centre for Medicinal Cannabis. H.S. is on the scientific advisory board of Cellen Inc. D.J.N. is chair of the charity Drug Science. A.K.S. is head of research of the charity Drug Science. Drug Science receives an unrestricted educational grant from a consortium of medical cannabis companies. C.S. was clinical director of Drug Science's Project Twenty21. G.Z. is chief scientific officer and scientific board member at Cannaray Ltd., advisory board member and teacher at Masterclass Medicinal Cannabis, and scientific board member at Portugal Medical Cannabis (PTMC). All other authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
