Signaling levels mold the RAS mutation tropism of urethane
- PMID: 33998997
- PMCID: PMC8128437
- DOI: 10.7554/eLife.67172
Signaling levels mold the RAS mutation tropism of urethane
Abstract
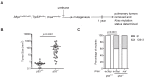
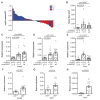
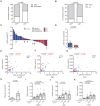
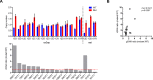
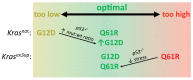
RAS genes are commonly mutated in human cancer. Despite many possible mutations, individual cancer types often have a 'tropism' towards a specific subset of RAS mutations. As driver mutations, these patterns ostensibly originate from normal cells. High oncogenic RAS activity causes oncogenic stress and different oncogenic mutations can impart different levels of activity, suggesting a relationship between oncoprotein activity and RAS mutation tropism. Here, we show that changing rare codons to common in the murine Kras gene to increase protein expression shifts tumors induced by the carcinogen urethane from arising from canonical Q61 to biochemically less active G12Kras driver mutations, despite the carcinogen still being biased towards generating Q61 mutations. Conversely, inactivating the tumor suppressor p53 to blunt oncogenic stress partially reversed this effect, restoring Q61 mutations. One interpretation of these findings is that the RAS mutation tropism of urethane arises from selection in normal cells for specific mutations that impart a narrow window of signaling that promotes proliferation without causing oncogenic stress.
Keywords: RAS; cancer biology; carcinogenesis; codon bias; mouse; oncogenesis; protooncogenes; tumor initiation.
© 2021, Li and Counter.
Conflict of interest statement
SL, CC No competing interests declared
Figures





Comment in
-
Danger zone.Elife. 2021 May 17;10:e69192. doi: 10.7554/eLife.69192. Elife. 2021. PMID: 33998996 Free PMC article.
References
-
- Bowling S, Sritharan D, Osorio FG, Nguyen M, Cheung P, Rodriguez-Fraticelli A, Patel S, Yuan WC, Fujiwara Y, Li BE, Orkin SH, Hormoz S, Camargo FD. An engineered CRISPR-Cas9 mouse line for simultaneous readout of lineage histories and gene expression profiles in single cells. Cell. 2020;181:1410–1422. doi: 10.1016/j.cell.2020.04.048. - DOI - PMC - PubMed
-
- Buffet C, Hecale-Perlemoine K, Bricaire L, Dumont F, Baudry C, Tissier F, Bertherat J, Cochand-Priollet B, Raffin-Sanson ML, Cormier F, Groussin L. DUSP5 and DUSP6, two ERK specific phosphatases, are markers of a higher MAPK signaling activation in BRAF mutated thyroid cancers. PLOS ONE. 2017;12:e0184861. doi: 10.1371/journal.pone.0184861. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

