The role of allergen-specific IgE, IgG and IgA in allergic disease
- PMID: 33999439
- PMCID: PMC8601105
- DOI: 10.1111/all.14908
The role of allergen-specific IgE, IgG and IgA in allergic disease
Abstract
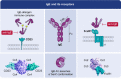
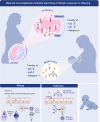
Immunoglobulin E (IgE)-mediated allergy is the most common hypersensitivity disease affecting more than 30% of the population. Exposure to even minute quantities of allergens can lead to the production of IgE antibodies in atopic individuals. This is termed allergic sensitization, which occurs mainly in early childhood. Allergen-specific IgE then binds to the high (FcεRI) and low-affinity receptors (FcεRII, also called CD23) for IgE on effector cells and antigen-presenting cells. Subsequent and repeated allergen exposure increases allergen-specific IgE levels and, by receptor cross-linking, triggers immediate release of inflammatory mediators from mast cells and basophils whereas IgE-facilitated allergen presentation perpetuates T cell-mediated allergic inflammation. Due to engagement of receptors which are highly selective for IgE, even tiny amounts of allergens can induce massive inflammation. Naturally occurring allergen-specific IgG and IgA antibodies usually recognize different epitopes on allergens compared with IgE and do not efficiently interfere with allergen-induced inflammation. However, IgG and IgA antibodies to these important IgE epitopes can be induced by allergen-specific immunotherapy or by passive immunization. These will lead to competition with IgE for binding with the allergen and prevent allergic responses. Similarly, anti-IgE treatment does the same by preventing IgE from binding to its receptor on mast cells and basophils. Here, we review the complex interplay of allergen-specific IgE, IgG and IgA and the corresponding cell receptors in allergic diseases and its relevance for diagnosis, treatment and prevention of allergy.
Keywords: IgE; allergy treatment; basic mechanisms in allergy; biologics; biomarkers; immunotherapy; tolerance induction; vaccines and mechanisms.
© 2021 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Shamji has nothing to disclose; Dr. Valenta reports grants and personal fees from Viravaxx, Vienna, Austria, grants from HVD Biotech, Vienna, Austria, grants and personal fees from WORG Pharmaceuticals, Hangzhou, China, outside the submitted work; Dr. Jardetzky reports other from Excellergy, Inc., outside the submitted work; In addition, Dr. Jardetzky has a patent covering novel anti‐IgE agents pending; Dr. Verhasselt has nothing to disclose; Dr. Durham has nothing to disclose; Dr. Würtzen is an employee of and owns stocks in ALK, a medical company producing allergy vaccines; Dr van Neerven has nothing to disclose.
Figures

References
-
- Ishizaka K, Ishizaka T, Hornbrook MM. Physico‐chemical properties of human reaginic antibody: IV. Presence of a unique immunoglobulin as a carrier of reaginic activity. J Immunol. 1966;97(1):75‐85. - PubMed
-
- Gould HJ, Sutton BJ, Beavil AJ, et al. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21(1):579‐628. - PubMed
-
- Pillai P, Fang C, Chan Y‐C, et al. Allergen‐specific IgE is not detectable in the bronchial mucosa of nonatopic asthmatic patients. Journal of Allergy and Clinical Immunology. 2014;133(6):1770–1772.e11. - PubMed
-
- Durham SR, Gould HJ, Hamid QA. Local IgE production in nasal allergy. Int Arch Allergy Immunol. 1997;113(1–3):128‐130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

