The Role of Cyclodextrins against Interface-Induced Denaturation in Pharmaceutical Formulations: A Molecular Dynamics Approach
- PMID: 33999634
- PMCID: PMC8289300
- DOI: 10.1021/acs.molpharmaceut.1c00135
The Role of Cyclodextrins against Interface-Induced Denaturation in Pharmaceutical Formulations: A Molecular Dynamics Approach
Abstract
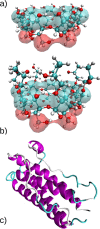
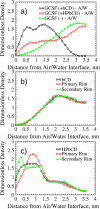
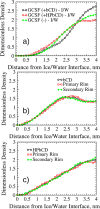
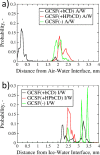
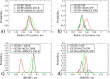
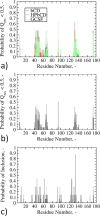
Protein-based pharmaceutical products are subject to a variety of environmental stressors, during both production and shelf-life. In order to preserve their structure, and, therefore, functionality, it is necessary to use excipients as stabilizing agents. Among the eligible stabilizers, cyclodextrins (CDs) have recently gained interest in the scientific community thanks to their properties. Here, a computational approach is proposed to clarify the role of β-cyclodextrin (βCD) and 2-hydroxypropyl-β-cyclodextrin (HPβCD) against granulocyte colony-stimulating (GCSF) factor denaturation at the air-water and ice-water interfaces, and also in bulk water at 300 or 260 K. Both traditional molecular dynamics (MD) simulations and enhanced sampling techniques (metadynamics, MetaD) are used to shed light on the underlying molecular mechanisms. Bulk simulations revealed that CDs were preferentially included within the surface hydration layer of GCSF, and even included some peptide residues in their hydrophobic cavity. HPβCD was able to stabilize the protein against surface-induced denaturation in proximity of the air-water interface, while βCD had a destabilizing effect. No remarkable conformational changes of GCSF, or noticeable effect of the CDs, were instead observed at the ice surface. GCSF seemed less stable at low temperature (260 K), which may be attributed to cold-denaturation effects. In this case, CDs did not significantly improve conformational stability. In general, the conformationally altered regions of GCSF seemed not to depend on the presence of excipients that only modulated the extent of destabilization with either a positive or a negative effect.
Keywords: cyclodextrins; denaturation; interface; molecular dynamics; protein stability.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Binary inclusion complexes of diflunisal with β-cyclodextrin and hydroxypropyl-β-cyclodextrin: Preparation and characterization.Pak J Pharm Sci. 2020 Sep;33(5(Supplementary)):2307-2315. Pak J Pharm Sci. 2020. PMID: 33832905
-
Inclusion complex and nanoclusters of cyclodextrin to increase the solubility and efficacy of albendazole.Parasitol Res. 2018 Mar;117(3):705-712. doi: 10.1007/s00436-017-5740-3. Epub 2018 Jan 11. Parasitol Res. 2018. PMID: 29327323 Clinical Trial.
-
Cytotoxicity of β-Cyclodextrins in Retinal Explants for Intravitreal Drug Formulations.Molecules. 2021 Mar 9;26(5):1492. doi: 10.3390/molecules26051492. Molecules. 2021. PMID: 33803405 Free PMC article.
-
Cyclodextrins--enabling excipients: their present and future use in pharmaceuticals.Crit Rev Ther Drug Carrier Syst. 1997;14(1):1-104. Crit Rev Ther Drug Carrier Syst. 1997. PMID: 9043816 Review.
-
Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization.J Pharm Sci. 1996 Oct;85(10):1017-25. doi: 10.1021/js950534b. J Pharm Sci. 1996. PMID: 8897265 Review.
Cited by
-
Combining Molecular Dynamics Simulations and Biophysical Characterization to Investigate Protein-Specific Excipient Effects on Reteplase during Freeze Drying.Pharmaceutics. 2023 Jun 30;15(7):1854. doi: 10.3390/pharmaceutics15071854. Pharmaceutics. 2023. PMID: 37514040 Free PMC article.
-
Force Field Parameterization for the Description of the Interactions between Hydroxypropyl-β-Cyclodextrin and Proteins.J Phys Chem B. 2021 Jul 15;125(27):7397-7405. doi: 10.1021/acs.jpcb.1c04033. Epub 2021 Jul 1. J Phys Chem B. 2021. PMID: 34210121 Free PMC article.
-
Predictive Model Building for Aggregation Kinetics Based on Molecular Dynamics Simulations of an Antibody Fragment.Mol Pharm. 2024 Nov 4;21(11):5827-5841. doi: 10.1021/acs.molpharmaceut.4c00859. Epub 2024 Sep 30. Mol Pharm. 2024. PMID: 39348223 Free PMC article.
-
Mechanistic Investigation of tert-Butanol's Impact on Biopharmaceutical Formulations: When Experiments Meet Molecular Dynamics.Mol Pharm. 2023 Aug 7;20(8):3975-3986. doi: 10.1021/acs.molpharmaceut.3c00125. Epub 2023 Jul 12. Mol Pharm. 2023. PMID: 37435823 Free PMC article.
-
Impact of Different Saccharides on the In-Process Stability of a Protein Drug During Evaporative Drying: From Sessile Droplet Drying to Lab-Scale Spray Drying.Pharm Res. 2023 May;40(5):1283-1298. doi: 10.1007/s11095-023-03498-w. Epub 2023 Apr 3. Pharm Res. 2023. PMID: 37012535 Free PMC article.
References
-
- Wang W.; Roberts C. J.. Aggregation of Therapeutic Proteins; John Wiley & Sons: 2010, 10.1002/9780470769829. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

