Updated Results of TBCRC026: Phase II Trial Correlating Standardized Uptake Value With Pathological Complete Response to Pertuzumab and Trastuzumab in Breast Cancer
- PMID: 33999652
- PMCID: PMC8260904
- DOI: 10.1200/JCO.21.00280
Updated Results of TBCRC026: Phase II Trial Correlating Standardized Uptake Value With Pathological Complete Response to Pertuzumab and Trastuzumab in Breast Cancer
Abstract
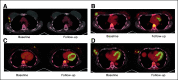
Purpose: Predictive biomarkers to identify patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer who may benefit from targeted therapy alone are required. We hypothesized that early measurements of tumor maximum standardized uptake value corrected for lean body mass (SULmax) on 18F-labeled fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) would predict pathologic complete response (pCR) to pertuzumab and trastuzumab (PT).
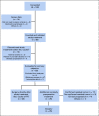
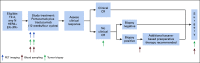
Patients and methods: Patients with stage II or III, estrogen receptor-negative, HER2-positive breast cancer received four cycles of neoadjuvant PT. 18F-labeled fluorodeoxyglucose positron emission tomography-computed tomography was performed at baseline and 15 days after PT initiation (C1D15). Eighty evaluable patients were required to test the null hypothesis that the area under the curve of percent change in SULmax by C1D15 predicting pCR is ≤ 0.65, with a one-sided type I error rate of 10%.
Results: Eighty-eight women were enrolled (83 evaluable), and 85% (75 of 88) completed all four cycles of PT. pCR after PT alone was 22%. Receiver operator characteristic analysis of percent change in SULmax by C1D15 yielded an area under the curve of 0.72 (80% CI, 0.64 to 0.80; one-sided P = .12), which did not reject the null hypothesis. However, between patients who obtained pCR and who did not, a significant difference in median percent reduction in SULmax by C1D15 was observed (63.8% v 41.8%; P = .004) and SULmax reduction ≥ 40% was more prevalent (83% v 52%; P = .03; positive predictive value, 31%). Participants not obtaining a 40% reduction in SULmax by C1D15 were unlikely to obtain pCR (negative predictive value, 91%).
Conclusion: Although the primary objective was not met, early changes in SULmax predict response to PT in estrogen receptor-negative and HER2-positive breast cancer. Once optimized, this quantitative imaging strategy may facilitate tailoring of therapy in this setting.
Conflict of interest statement
Figures


Comment in
-
18F-FDG PET/CT Predicts Response to HER2-directed Neoadjuvant Therapy.Radiol Imaging Cancer. 2021 Sep;3(5):e219021. doi: 10.1148/rycan.2021219021. Radiol Imaging Cancer. 2021. PMID: 34533375 Free PMC article. No abstract available.
References
-
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial Lancet Oncol 1325–322012 - PubMed
-
- Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol 242278–22842013 - PubMed
-
- Prowell TM, Pazdur R.Pathological complete response and accelerated drug approval in early breast cancer N Engl J Med 3662438–24412012 - PubMed
-
- Nitz UA, Gluz O, Christgen M, et al. De-escalation strategies in HER2-positive early breast cancer (EBC): Final analysis of the WSG-ADAPT HER2+/HR− phase II trial: Efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab ± weekly paclitaxel Ann Oncol 282768–27722017 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

