Does varying the ingestion period of sodium citrate influence blood alkalosis and gastrointestinal symptoms?
- PMID: 33999939
- PMCID: PMC8128256
- DOI: 10.1371/journal.pone.0251808
Does varying the ingestion period of sodium citrate influence blood alkalosis and gastrointestinal symptoms?
Abstract
Objectives: To compare blood alkalosis, gastrointestinal symptoms and indicators of strong ion difference after ingestion of 500 mg.kg-1 BM sodium citrate over four different periods.
Methods: Sixteen healthy and active participants ingested 500 mg.kg-1 BM sodium citrate in gelatine capsules over a 15, 30, 45 or 60 min period using a randomized cross-over experimental design. Gastrointestinal symptoms questionnaires and venous blood samples were collected before ingestion, immediately post-ingestion, and every 30 min for 480 min post-ingestion. Blood samples were analysed for blood pH, [HCO3-], [Na+], [Cl-] and plasma [citrate]. Linear mixed models were used to estimate the effect of the ingestion protocols.
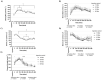
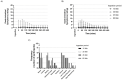
Results: For all treatments, blood [HCO3-] was significantly elevated above baseline for the entire 480 min post-ingestion period, and peak occurred 180 min post-ingestion. Blood [HCO3-] and pH were significantly elevated above baseline and not significantly below the peak between 150-270 min post-ingestion. Furthermore, blood pH and [HCO3-] were significantly lower for the 60 min ingestion period when compared to the other treatments. Gastrointestinal symptoms were minor for all treatments; the mean total session symptoms ratings (all times summed together) were between 9.8 and 11.6 from a maximum possible rating of 720.
Conclusion: Based on the findings of this investigation, sodium citrate should be ingested over a period of less than 60 min (15, 30 or 45 min), and completed 150-270 min before exercise.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

