Novel perspective on a conventional technique: Impact of ultra-low temperature on bacterial viability and protein extraction
- PMID: 33999956
- PMCID: PMC8128238
- DOI: 10.1371/journal.pone.0251640
Novel perspective on a conventional technique: Impact of ultra-low temperature on bacterial viability and protein extraction
Abstract
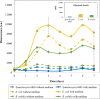
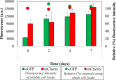
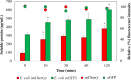
Ultra-low temperature (ULT) storage of microbial biomass is routinely practiced in biological laboratories. However, there is very little insight regarding the effects of biomass storage at ULT and the structure of the cell envelope, on cell viability. Eventually, these aspects influence bacterial cell lysis which is one of the critical steps for biomolecular extraction, especially protein extraction. Therefore, we studied the effects of ULT-storage (-80°C) on three different bacterial platforms: Escherichia coli, Bacillus subtilis and the cyanobacterium Synechocystis sp. PCC 6803. By using a propidium iodide assay and a modified MTT assay we determined the impact of ULT storage on cellular viability. Subsequently, the protein extraction efficiency was determined by analyzing the amount of protein released following the storage. The results successfully established that longer the ULT-storage time lower is the cell viability and larger is the protein extraction efficiency. Interestingly, E. coli and B. subtilis exhibited significant reduction in cell viability over Synechocystis 6803. This indicates that the cell membrane structure and composition may play a major role on cell viability in ULT storage. Interestingly, E. coli exhibited concomitant increase in cell lysis efficiency resulting in a 4.5-fold increase (from 109 μg/ml of protein on day 0 to 464 μg/ml of protein on day 2) in the extracted protein titer following ULT storage. Furthermore, our investigations confirmed that the protein function, tested through the extraction of fluorescent proteins from cells stored at ULT, remained unaltered. These results established the plausibility of using ULT storage to improve protein extraction efficiency. Towards this, the impact of shorter ULT storage time was investigated to make the strategy more time efficient to be adopted into protocols. Interestingly, E. coli transformants expressing mCherry yielded 2.7-fold increase (93 μg/mL to 254 μg/mL) after 10 mins, while 4-fold increase (380 μg/mL) after 120 mins of ULT storage in the extracted soluble protein. We thereby substantiate that: (1) the storage time of bacterial cells in -80°C affect cell viability and can alter protein extraction efficiency; and (2) exercising a simple ULT-storage prior to bacterial cell lysis can improve the desired protein yield without impacting its function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Sarnaik A, Sawant K, Khadilkar J, Pillai G, Pandit R, Lali A. Cyanobacterial Cell Factories for Improved Carotenoid Biosynthesis through a Synthetic Biology Approach. Next Generation Biomanufacturing Technologies. ACS Symposium Series. 1329: American Chemical Society; 2019. p. 23–39.
-
- Sarnaik A, Nambissan V, Pandit R, Lali A. Recombinant Synechococcus elongatus PCC 7942 for improved zeaxanthin production under natural light conditions. Algal Research. 2018;36:139–51.
-
- Gao W, Leung K, Hawdon N. Freezing inactivation of Escherichia coli and Enterococcus faecalis in water: response of different strains. Water Environ Res. 2009;81(8):824–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

