Assessment of a recombinant protein from Leishmania infantum as a novel tool for Visceral Leishmaniasis (VL) diagnosis in VL/HIV co-infection cases
- PMID: 33999968
- PMCID: PMC8128258
- DOI: 10.1371/journal.pone.0251861
Assessment of a recombinant protein from Leishmania infantum as a novel tool for Visceral Leishmaniasis (VL) diagnosis in VL/HIV co-infection cases
Abstract
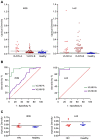
Visceral Leishmaniasis and HIV-AIDS coinfection (VL/HIV) is considered a life-threatening pathology when undiagnosed and untreated, due to the immunosuppression caused by both diseases. Serological tests largely used for the VL diagnosis include the direct agglutination test (DAT), ELISA and immunochromatographic (ICT) assays. For VL diagnosis in HIV infections, different studies have shown that the use of the DAT assay facilitates the VL diagnosis in co-infected patients, since the performance of the most widely used ELISA and ICT tests, based on the recombinant protein rK39, are much less efficient in HIV co-infections. In this scenario, alternative recombinant antigens may help the development of new serological diagnostic methods which may improve the VL diagnosis for the co-infection cases. This work aimed to evaluate the use of the recombinant Lci2 antigen, related to, but antigenically more diverse than rK39, for VL diagnosis in co-infected sera through ELISA assays. A direct comparison between recombinant Lci2 and rK39 was thus carried out. The two proteins were first tested using indirect ELISA with sera from VL afflicted individuals and healthy controls, with similar performances. They were then tested with two different sets of VL/HIV co-infected cases and a significant drop in performance, for one of these groups, was observed for rK39 (32% sensitivity), but not for Lci2 (98% sensitivity). In fact, an almost perfect agreement (Kappa: 0.93) between the Lci2 ELISA and DAT was observed for the coinfected VL/HIV patients. Lci2 then has the potential to be used as a new tool for the VL diagnosis of VL/HIV co-infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

