Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial
- PMID: 34000236
- PMCID: PMC8121541
- DOI: 10.1016/S2213-2600(21)00177-6
Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial
Erratum in
-
Correction to Lancet Respir Med 2021; published online May 14. https://doi.org/10.1016/S2213-2600(21)00177-6.Lancet Respir Med. 2021 Jul;9(7):e62. doi: 10.1016/S2213-2600(21)00273-3. Epub 2021 Jun 4. Lancet Respir Med. 2021. PMID: 34097911 Free PMC article. No abstract available.
Abstract
Background: We sought to clarify the benefit of cytokine adsorption in patients with COVID-19 supported with venovenous extracorporeal membrane oxygenation (ECMO).
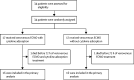
Methods: We did a single-centre, open-label, randomised, controlled trial to investigate cytokine adsorption in adult patients with severe COVID-19 pneumonia requiring ECMO. Patients with COVID-19 selected for ECMO at the Freiburg University Medical Center (Freiburg, Germany) were randomly assigned (1:1) to receive cytokine adsorption using the CytoSorb device or not. Randomisation was computer-generated, allocation was concealed by opaque, sequentially numbered sealed envelopes. The CytoSorb device was incorporated into the ECMO circuit before connection to the patient circuit, replaced every 24 h, and removed after 72 h. The primary endpoint was serum interleukin-6 (IL-6) concentration 72 h after initiation of ECMO analysed by intention to treat. Secondary endpoints included 30-day survival. The trial is registered with ClinicalTrials.gov (NCT04324528) and the German Clinical Trials Register (DRKS00021300) and is closed.
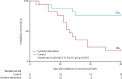
Findings: From March 29, 2020, to Dec 29, 2020, of 34 patients assessed for eligibility, 17 (50%) were treated with cytokine adsorption and 17 (50%) without. Median IL-6 decreased from 357·0 pg/mL to 98·6 pg/mL in patients randomly assigned to cytokine adsorption and from 289·0 pg/mL to 112·0 pg/mL in the control group after 72 h. One patient in each group died before 72 h. Adjusted mean log IL-6 concentrations after 72 h were 0·30 higher in the cytokine adsorption group (95% CI -0·70 to 1·30, p=0·54). Survival after 30 days was three (18%) of 17 with cytokine adsorption and 13 (76%) of 17 without cytokine adsorption (p=0·0016).
Interpretation: Early initiation of cytokine adsorption in patients with severe COVID-19 and venovenous ECMO did not reduce serum IL-6 and had a negative effect on survival. Cytokine adsorption should not be used during the first days of ECMO support in COVID-19.
Funding: None.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declarations of interests All authors have completed the ICMJE form (available on request from the corresponding author). ASu reports research grants and lecture fees from CytoSorbents and lecture fees from Abiomed, both outside of the submitted work. MR reports funding by the IMM-PACT-Programme for Clinician Scientists, Department of Medicine II, Medical Center, University of Freiburg and Faculty of Medicine, funded by the Deutsche Forschungsgemeinschaft (German Research Foundation)—413517907 and financial support from CytoSorbents for attending a scientific meeting. AL reports a research grant from the German Center for infectious diseases, outside of the submitted work and is a member of SFB1425, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation #422681845). SMa reports honoraria from CytoSorbents for a presentation during a scientific workshop. CBe and GT are shareholders of Resuscitec, they received personal fees from Resuscitec and they hold patents US 10695407 and EU 3016675 issued to Resuscitec. GT reports grants from Resuscitec and payments from Medela, both outside of the submitted work. CBe reports lecture honoraria from CytoSorbents. DS reports lecture fees from Orion Pharma, Abiomed, Getinge Group, AstraZeneca, MedCaptain, and Medtronic, travel support for the attendance of scientific meetings from Orion Pharma and Abiomed, and fees from Orion Pharma for medical writing work. EG reports consulting fees from Roche Pharma, Germany, for statistical consulting, outside of the submitted work. DD reports research grants, lecture fees and travel support from CytoSorbents, all outside of the submitted work and is a member of SFB1425, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation #422681845). All other authors declare no conflicts of interest.
Figures

Comment in
-
Cytokine adsorption during ECMO for COVID-19-related ARDS.Lancet Respir Med. 2021 Jul;9(7):680-682. doi: 10.1016/S2213-2600(21)00207-1. Epub 2021 May 14. Lancet Respir Med. 2021. PMID: 34000235 Free PMC article. No abstract available.
-
Cytokine adsorption and ECMO in patients with COVID-19.Lancet Respir Med. 2021 Aug;9(8):e69-e70. doi: 10.1016/S2213-2600(21)00276-9. Epub 2021 Jul 6. Lancet Respir Med. 2021. PMID: 34242579 Free PMC article. No abstract available.
-
Cytokine adsorption and ECMO in patients with COVID-19 - Author's reply.Lancet Respir Med. 2021 Aug;9(8):e72-e74. doi: 10.1016/S2213-2600(21)00274-5. Epub 2021 Jul 6. Lancet Respir Med. 2021. PMID: 34242580 Free PMC article. No abstract available.
-
Cytokine adsorption and ECMO in patients with COVID-19.Lancet Respir Med. 2021 Aug;9(8):e71. doi: 10.1016/S2213-2600(21)00285-X. Epub 2021 Jul 6. Lancet Respir Med. 2021. PMID: 34242581 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

