Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial
- PMID: 34000257
- PMCID: PMC8121538
- DOI: 10.1016/S0140-6736(21)00897-7
Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial
Abstract
Background: Many patients with COVID-19 have been treated with plasma containing anti-SARS-CoV-2 antibodies. We aimed to evaluate the safety and efficacy of convalescent plasma therapy in patients admitted to hospital with COVID-19.
Methods: This randomised, controlled, open-label, platform trial (Randomised Evaluation of COVID-19 Therapy [RECOVERY]) is assessing several possible treatments in patients hospitalised with COVID-19 in the UK. The trial is underway at 177 NHS hospitals from across the UK. Eligible and consenting patients were randomly assigned (1:1) to receive either usual care alone (usual care group) or usual care plus high-titre convalescent plasma (convalescent plasma group). The primary outcome was 28-day mortality, analysed on an intention-to-treat basis. The trial is registered with ISRCTN, 50189673, and ClinicalTrials.gov, NCT04381936.
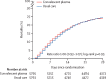
Findings: Between May 28, 2020, and Jan 15, 2021, 11558 (71%) of 16287 patients enrolled in RECOVERY were eligible to receive convalescent plasma and were assigned to either the convalescent plasma group or the usual care group. There was no significant difference in 28-day mortality between the two groups: 1399 (24%) of 5795 patients in the convalescent plasma group and 1408 (24%) of 5763 patients in the usual care group died within 28 days (rate ratio 1·00, 95% CI 0·93-1·07; p=0·95). The 28-day mortality rate ratio was similar in all prespecified subgroups of patients, including in those patients without detectable SARS-CoV-2 antibodies at randomisation. Allocation to convalescent plasma had no significant effect on the proportion of patients discharged from hospital within 28 days (3832 [66%] patients in the convalescent plasma group vs 3822 [66%] patients in the usual care group; rate ratio 0·99, 95% CI 0·94-1·03; p=0·57). Among those not on invasive mechanical ventilation at randomisation, there was no significant difference in the proportion of patients meeting the composite endpoint of progression to invasive mechanical ventilation or death (1568 [29%] of 5493 patients in the convalescent plasma group vs 1568 [29%] of 5448 patients in the usual care group; rate ratio 0·99, 95% CI 0·93-1·05; p=0·79).
Interpretation: In patients hospitalised with COVID-19, high-titre convalescent plasma did not improve survival or other prespecified clinical outcomes.
Funding: UK Research and Innovation (Medical Research Council) and National Institute of Health Research.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures


Comment in
-
Convalescent plasma in patients hospitalised with COVID-19.Lancet. 2021 May 29;397(10289):2024-2025. doi: 10.1016/S0140-6736(21)01064-3. Epub 2021 May 14. Lancet. 2021. PMID: 34000255 Free PMC article. No abstract available.
-
In patients hospitalized with COVID-19, adding convalescent plasma to usual care did not reduce 28-d mortality.Ann Intern Med. 2021 Oct;174(10):JC113. doi: 10.7326/ACPJ202110190-113. Epub 2021 Oct 5. Ann Intern Med. 2021. PMID: 34606320
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 16896/CRUK_/Cancer Research UK/United Kingdom
- MR/S001751/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00002/14/MRC_/Medical Research Council/United Kingdom
- MC_U137686861/MRC_/Medical Research Council/United Kingdom
- G0701652/MRC_/Medical Research Council/United Kingdom
- MR/K025643/1/MRC_/Medical Research Council/United Kingdom
- 211153/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- MC_U137686860/MRC_/Medical Research Council/United Kingdom
- 25350/CRUK_/Cancer Research UK/United Kingdom
- MC_UU_12026/4/MRC_/Medical Research Council/United Kingdom
- CS/18/2/33719/BHF_/British Heart Foundation/United Kingdom
- MC_PC_20062/MRC_/Medical Research Council/United Kingdom
- MC_PC_19056/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

