Causal relationship of CA3 back-projection to the dentate gyrus and its role in CA1 fast ripple generation
- PMID: 34001031
- PMCID: PMC8130286
- DOI: 10.1186/s12868-021-00641-4
Causal relationship of CA3 back-projection to the dentate gyrus and its role in CA1 fast ripple generation
Abstract
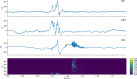
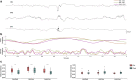
Background: Pathophysiological evidence from temporal lobe epilepsy models highlights the hippocampus as the most affected structure due to its high degree of neuroplasticity and control of the dynamics of limbic structures, which are necessary to encode information, conferring to it an intrinsic epileptogenicity. A loss in this control results in observable oscillatory perturbations called fast ripples, in epileptic rats those events are found in CA1, CA3, and the dentate gyrus (DG), which are the principal regions of the trisynaptic circuit of the hippocampus. The present work used Granger causality to address which relationships among these three regions of the trisynaptic circuit are needed to cause fast ripples in CA1 in an in vivo model. For these purposes, male Wistar rats (210-300 g) were injected with a single dose of pilocarpine hydrochloride (2.4 mg/2 µl) into the right lateral ventricle and video-monitored 24 h/day to detect spontaneous and recurrent seizures. Once detected, rats were implanted with microelectrodes in these regions (fixed-recording tungsten wire electrodes, 60-μm outer diameter) ipsilateral to the pilocarpine injection. A total of 336 fast ripples were recorded and probabilistically characterized, from those fast ripples we made a subset of all the fast ripple events associated with sharp-waves in CA1 region (n = 40) to analyze them with Granger Causality.
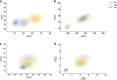
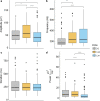
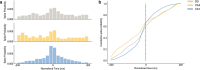
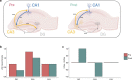
Results: Our results support existing evidence in vitro in which fast ripple events in CA1 are initiated by CA3 multiunit activity and describe a general synchronization in the theta band across the three regions analyzed DG, CA3, and CA1, just before the fast ripple event in CA1 have begun.
Conclusion: This in vivo study highlights the causal participation of the CA3 back-projection to the DG, a connection commonly overlooked in the trisynaptic circuit, as a facilitator of a closed-loop among these regions that prolongs the excitatory activity of CA3. We speculate that the loss of inhibitory drive of DG and the mechanisms of ripple-related memory consolidation in which also the CA3 back-projection to DG has a fundamental role might be underlying processes of the fast ripples generation in CA1.
Keywords: Fast ripples; Granger causality; Hippocampus; In vivo studies; Pilocarpine model; Theta rhythm.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Evaluation of the hippocampal immunoreactivity of the serotonin 5-HT1A, 5-HT2 and 5-HT7 receptors in a pilocarpine temporal lobe epilepsy rat model with fast ripples.Neuroreport. 2021 Mar 3;32(4):306-311. doi: 10.1097/WNR.0000000000001594. Neuroreport. 2021. PMID: 33470771
-
Preictal activity of subicular, CA1, and dentate gyrus principal neurons in the dorsal hippocampus before spontaneous seizures in a rat model of temporal lobe epilepsy.J Neurosci. 2014 Dec 10;34(50):16671-87. doi: 10.1523/JNEUROSCI.0584-14.2014. J Neurosci. 2014. PMID: 25505320 Free PMC article.
-
The hippocampal CA3 region can generate two distinct types of sharp wave-ripple complexes, in vitro.Hippocampus. 2015 Feb;25(2):169-86. doi: 10.1002/hipo.22361. Epub 2014 Sep 25. Hippocampus. 2015. PMID: 25209976
-
Function of local circuits in the hippocampal dentate gyrus-CA3 system.Neurosci Res. 2019 Mar;140:43-52. doi: 10.1016/j.neures.2018.11.003. Epub 2018 Nov 5. Neurosci Res. 2019. PMID: 30408501 Review.
-
Functional optical probing of the hippocampal trisynaptic circuit in vitro: network dynamics, filter properties, and polysynaptic induction of CA1 LTP.Front Neurosci. 2015 May 6;9:160. doi: 10.3389/fnins.2015.00160. eCollection 2015. Front Neurosci. 2015. PMID: 25999809 Free PMC article. Review.
Cited by
-
GABAA Receptor-Stabilizing Protein Ubqln1 Affects Hyperexcitability and Epileptogenesis after Traumatic Brain Injury and in a Model of In Vitro Epilepsy in Mice.Int J Mol Sci. 2022 Mar 31;23(7):3902. doi: 10.3390/ijms23073902. Int J Mol Sci. 2022. PMID: 35409261 Free PMC article.
-
Pattern Separation and Pattern Completion Within the Hippocampal Circuit During Naturalistic Stimuli.Hum Brain Mapp. 2025 Feb 1;46(2):e70150. doi: 10.1002/hbm.70150. Hum Brain Mapp. 2025. PMID: 39878229 Free PMC article.
-
Highly dynamic inflammatory and excitability transcriptional profiles in hippocampal CA1 following status epilepticus.Sci Rep. 2023 Dec 14;13(1):22187. doi: 10.1038/s41598-023-49310-y. Sci Rep. 2023. PMID: 38092829 Free PMC article.
-
Epileptiform activity in mouse hippocampal slices induced by moderate changes in extracellular Mg2+, Ca2+, and K.BMC Neurosci. 2021 Jul 23;22(1):46. doi: 10.1186/s12868-021-00650-3. BMC Neurosci. 2021. PMID: 34301200 Free PMC article.
References
-
- Angus-Leppan H, Parsons LM. Epilepsy: epidemiology, classification and natural history. Medicine (Baltimore) 2008;36:571–578. doi: 10.1016/j.mpmed.2008.08.003. - DOI
-
- Cendes F. Mesial temporal lobe epilepsy syndrome: an updated overview. J Epilepsy Clin Neurophysiol. 2005;11:141–144. doi: 10.1590/S1676-26492005000300006. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

