MR-guided microwave ablation of hepatocellular carcinoma (HCC): is general anesthesia more effective than local anesthesia?
- PMID: 34001036
- PMCID: PMC8130145
- DOI: 10.1186/s12885-021-08298-2
MR-guided microwave ablation of hepatocellular carcinoma (HCC): is general anesthesia more effective than local anesthesia?
Abstract
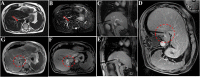
Background: Percutaneous magnetic resonance-guided (MR-guided) MWA procedures have traditionally been performed under local anesthesia (LA) and sedation. However, pain control is often difficult to manage, especially in some cases when the tumor is large or in a specific location, such as near the abdominal wall or close to the hepatic dome. This study retrospectively compared the results of general anesthesia (GA) and local anesthesia (LA) for MR-guided microwave ablation (MWA) in patients with hepatocellular carcinoma (HCC ≤ 5.0 cm) to investigate whether different anesthesia methods lead to different clinical outcomes.
Methods: The results of the analysis include procedure-related complications, imaging response, and the time to complete two sets of procedures. According to the type of anesthesia, the Kaplan-Meier method was used to compare the local tumor progression (LTP) of the two groups who underwent MR-guided MWA.
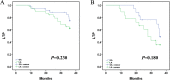
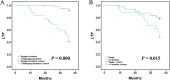
Results: All patients achieved technical success. The mean ablation duration of each patient in the GA group and LA group was remarkably different (P = 0.012). Both groups had no difference in complications or LTP (both P > 0.05). Notably, the tumor location (challenging locations) and the number of lesions (2-3 lesions) could be the main factors affecting LTP (p = 0.000, p = 0.015). Univariate Cox proportional hazard regression indicated that using different anesthesia methods (GA and LA) was not associated with longer LTP (P = 0.237), while tumor location (challenging locations) and the number of lesions (2-3 lesions) were both related to shorter LTP (P = 0.000, P = 0.020, respectively). Additionally, multivariate Cox regression further revealed that the tumor location (regular locations) and the number of lesions (single) could independently predict better LTP (P = 0.000, P = 0.005, respectively).
Conclusions: No correlation was observed between GA and LA for LTP after MR-guided MWA. However, tumors in challenging locations and the number of lesions (2-3 lesions) appear to be the main factors affecting LTP.
Keywords: Hepatocellular carcinoma; Interventional radiology; Magnetic resonance imaging; Microwave ablation.
Conflict of interest statement
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Figures

References
-
- Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, de Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, van Hoek B, Majno P, Metroticket Investigator Study Group Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35–43. doi: 10.1016/S1470-2045(08)70284-5. - DOI - PubMed
-
- Dou JP, Han ZY, Cheng ZG, et al. The effect of tumor location on long-term results of microwave ablation for early-stage hepatocellular carcinoma. Abdom Radiol (NY). 2020. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

