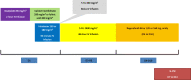
FOLFIRINOX-R study design: a phase I/II trial of FOLFIRINOX plus regorafenib as first line therapy in patients with unresectable RAS-mutated metastatic colorectal cancer
- PMID: 34001059
- PMCID: PMC8130420
- DOI: 10.1186/s12885-021-08312-7
FOLFIRINOX-R study design: a phase I/II trial of FOLFIRINOX plus regorafenib as first line therapy in patients with unresectable RAS-mutated metastatic colorectal cancer
Abstract
Background: The chemotherapy triplet FOLFOXIRI combined to the anti-VEGF antibody bevacizumab is an option in selected patients with metastatic colorectal cancer. In this setting, RAS-mutated metastatic colorectal cancer do not benefit the same from treatment than RAS-wildtype metastatic colorectal cancer do. Together with its antiangiogenic properties, the tyrosine-kinase inhibitor regorafenib has also anti-proliferative activities whatever the RAS status is. The present trial aims at studying the safety and the efficacy of regorafenib in combination with FOLFIRINOX - a chemotherapy triplet using a different dosing schedule than FOLFOXIRI - in patients with RAS-mutated metastatic colorectal cancer.
Methods: FOLFIRINOX-R is a prospective, multicentric, non-randomised, dose-finding phase 1-2 trial. The primary endpoints are the determination of the maximum tolerated dose, the recommended phase 2 dose, and the proportion of patients achieving disease control at 48-weeks. Phase 1 follows a 3 + 3 design (12 to 24 patients to be included). Sixty nine patients will be necessary in phase 2, including 5% non-evaluable ones, with the following assumptions, one-stage Fleming design, α = 5%, β = 20%, p0 = 35% and p1 = 50%. Key eligibility criteria include Eastern Cooperative Oncology Group Performance Status of ≤1 and RAS-mutated metastatic colorectal cancer not amenable to surgery with curative intent and not previously treated for metastatic disease. FOLFIRINOX (oxaliplatin 85 mg/m2, folinic acid 400 mg/m2, irinotecan 150-180 mg/m2, 5-fluorouracil: 400 mg/m2 then 2400 mg/m2 over 46 h) is administered every 14 days. Regorafenib (80 to 160 mg, as per dose-level) is administered orally, once daily on days 4 to 10 of each cycle.
Discussion: FOLFIRINOX-R is the first phase I/II study to evaluate the safety and efficacy of regorafenib in combination with FOLFIRINOX as frontline therapy for patients with RAS-mutated metastatic colorectal cancer.
Trial registration: EudraCT: 2018-003541-42 ; ClinicalTrials.gov: NCT03828799 .
Keywords: Chemotherapy triplet; Colorectal cancer; Phase 1–2 trial; Regorafenib.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R, Ferlay J, editors. Cancer Incidence in Five Continents, Vol. XI. IARC Scientific Publication No. 166. Lyon: International Agency for Research on Cancer; 2020. Available from: https://publications.iarc.fr/597.
-
- Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, Chiara S, Granetto C, Porcile G, Fioretto L, Orlandini C, Andreuccetti M, Masi G, Gruppo Oncologico Nord Ovest Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1670–1676. doi: 10.1200/JCO.2006.09.0928. - DOI - PubMed
-
- Ychou M, Rivoire M, Thezenas S, Quenet F, Delpero JR, Rebischung C, Letoublon C, Guimbaud R, Francois E, Ducreux M, Desseigne F, Fabre JM, Assenat E. A randomized phase II trial of three intensified chemotherapy regimens in first-line treatment of colorectal cancer patients with initially unresectable or not optimally resectable liver metastases. The METHEP trial. Ann Surg Oncol. 2013;20(13):4289–4297. doi: 10.1245/s10434-013-3217-x. - DOI - PubMed
-
- Modest DP, Ricard I, Heinemann V, Hegewisch-Becker S, Schmiegel W, Porschen R, Stintzing S, Graeven U, Arnold D, von Weikersthal LF, Giessen-Jung C, Stahler A, Schmoll HJ, Jung A, Kirchner T, Tannapfel A, Reinacher-Schick A. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol. 2016;27(9):1746–1717. doi: 10.1093/annonc/mdw261. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical