Evaluation of a serum-based antigen test for tuberculosis in HIV-exposed infants: a diagnostic accuracy study
- PMID: 34001096
- PMCID: PMC8130139
- DOI: 10.1186/s12916-021-01983-w
Evaluation of a serum-based antigen test for tuberculosis in HIV-exposed infants: a diagnostic accuracy study
Abstract
Background: Non-sputum methods are urgently needed to improve tuberculosis diagnosis and treatment monitoring in children. This study evaluated the ability of a serum assay quantifying a species-specific peptide of the Mycobacterium tuberculosis CFP-10 virulence factor via nanotechnology and matrix-assisted laser desorption ionization time-of-flight mass spectrometry to diagnose tuberculosis in HIV-infected and HIV-uninfected infants.
Methods: Serum CFP-10 peptide signal was blinded evaluated in cryopreserved sera of 519 BCG-immunized, HIV-exposed infants (284 HIV-infected, 235 HIV-uninfected) from a multi-center randomized placebo-controlled isoniazid prophylaxis trial conducted in southern Africa between 2004 and 2008, who were followed up to 192 weeks for Mtb infection and TB. Children were classified as confirmed, unconfirmed, or unlikely tuberculosis cases using 2015 NIH diagnostic criteria for pediatric TB.
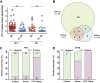
Results: In HIV-infected infants, CFP-10 signal had 100% sensitivity for confirmed TB (5/5, 95% CI, 47.8-100) and 83.7% sensitivity for unconfirmed TB (36/43, 95% CI 69.3-93.2), with 93.1% specificity (203/218, 95% CI 88.9-96.1). In HIV-uninfected infants, CFP-10 signal detected the single confirmed TB case and 75.0% of unconfirmed TB cases (15/20; 95% CI 50.9-91.3), with 96.2% specificity (177/184, 95% CI, 92.3-98.5). Serum CFP-10 achieved 77% diagnostic sensitivity for confirmed and unconfirmed TB (13/17, 95% CI, 50-93%) at ≤ 24 weeks pre-diagnosis, and both CFP-10-positivity and concentration declined following anti-TB therapy initiation.
Conclusions: Serum CFP-10 signal exhibited high diagnostic sensitivity and specificity for tuberculosis in HIV-infected and HIV-uninfected infants and potential utility for early TB detection and monitoring of anti-TB treatment responses.
Keywords: CFP-10; Mass spectrometry; Nanotechnology; Pediatric tuberculosis.
Conflict of interest statement
Y. H, C.L. and C.J.L. report other support from NanoPin Technologies, Inc., outside the submitted work; In addition, Y. H. has a patent “Compositions and methods of determining a level of active
Figures

References
-
- World Health Organization . Global tuberculosis report. 2019.
-
- Edwards DJ, Kitetele F, Van Rie A. Agreement between clinical scoring systems used for the diagnosis of pediatric tuberculosis in the HIV era. Int J Tuberc Lung Dis. 2007;11:263–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

