Design considerations of an IL13Rα2 antibody-drug conjugate for diffuse intrinsic pontine glioma
- PMID: 34001278
- PMCID: PMC8127302
- DOI: 10.1186/s40478-021-01184-9
Design considerations of an IL13Rα2 antibody-drug conjugate for diffuse intrinsic pontine glioma
Erratum in
-
Correction to: Design considerations of an IL13Rα2 antibody-drug conjugate for difuse intrinsic pontine glioma.Acta Neuropathol Commun. 2021 Jun 25;9(1):114. doi: 10.1186/s40478-021-01210-w. Acta Neuropathol Commun. 2021. PMID: 34172087 Free PMC article. No abstract available.
Abstract
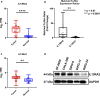
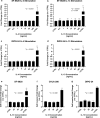
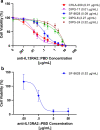
Diffuse intrinsic pontine glioma (DIPG), a rare pediatric brain tumor, afflicts approximately 350 new patients each year in the United States. DIPG is noted for its lethality, as fewer than 1% of patients survive to five years. Multiple clinical trials involving chemotherapy, radiotherapy, and/or targeted therapy have all failed to improve clinical outcomes. Recently, high-throughput sequencing of a cohort of DIPG samples identified potential therapeutic targets, including interleukin 13 receptor subunit alpha 2 (IL13Rα2) which was expressed in multiple tumor samples and comparably absent in normal brain tissue, identifying IL13Rα2 as a potential therapeutic target in DIPG. In this work, we investigated the role of IL13Rα2 signaling in progression and invasion of DIPG and viability of IL13Rα2 as a therapeutic target through the use of immunoconjugate agents. We discovered that IL13Rα2 stimulation via canonical ligands demonstrates minimal impact on both the cellular proliferation and cellular invasion of DIPG cells, suggesting IL13Rα2 signaling is non-essential for DIPG progression in vitro. However, exposure to an anti-IL13Rα2 antibody-drug conjugate demonstrated potent pharmacological response in DIPG cell models both in vitro and ex ovo in a manner strongly associated with IL13Rα2 expression, supporting the potential use of targeting IL13Rα2 as a DIPG therapy. However, the tested ADC was effective in most but not all cell models, thus selection of the optimal payload will be essential for clinical translation of an anti-IL13Rα2 ADC for DIPG.
Keywords: Antibody–drug conjugates; Diffuse intrinsic pontine glioma; IL13Rα2; Immunotherapy; Pediatric cancer.
Conflict of interest statement
Author CK is a co-founder of Artisan Biopharma, a wholly-owned public benefit corporation of the Children’s Cancer Therapy Development Institute (cc-TDI). CK through cc-TDI also has research frameworks or collaborations with Roche Genentech, Eli Lilly, and Novartis. The chorioallantoic membrane (CAM) assay process is being developed for patenting and commercialization by authors CK and SR. The remaining authors declare no competing interests.
Figures
Similar articles
-
Identification of new therapeutic targets and natural compounds against diffuse intrinsic pontine glioma (DIPG).Bioorg Chem. 2020 Jun;99:103847. doi: 10.1016/j.bioorg.2020.103847. Epub 2020 Apr 13. Bioorg Chem. 2020. PMID: 32311581 Free PMC article.
-
Identification of Novel RAS Signaling Therapeutic Vulnerabilities in Diffuse Intrinsic Pontine Gliomas.Cancer Res. 2019 Aug 15;79(16):4026-4041. doi: 10.1158/0008-5472.CAN-18-3521. Epub 2019 Jun 14. Cancer Res. 2019. PMID: 31201162
-
Targeting the SP/KLF Transcriptional Regulatory Network Synergizes with HDAC Inhibition to Impede Progression of H3K27M Diffuse Intrinsic Pontine Glioma.Cancer Res. 2025 Jun 2;85(11):2100-2116. doi: 10.1158/0008-5472.CAN-24-2227. Cancer Res. 2025. PMID: 40053472
-
Diffuse intrinsic pontine glioma (DIPG): A review of current and emerging treatment strategies.Cancer Lett. 2024 May 28;590:216876. doi: 10.1016/j.canlet.2024.216876. Epub 2024 Apr 10. Cancer Lett. 2024. PMID: 38609002 Free PMC article. Review.
-
Current status and advances to improving drug delivery in diffuse intrinsic pontine glioma.J Control Release. 2024 Jun;370:835-865. doi: 10.1016/j.jconrel.2024.05.018. Epub 2024 May 20. J Control Release. 2024. PMID: 38744345 Review.
Cited by
-
Immunogenic Cell Death Enhances Immunotherapy of Diffuse Intrinsic Pontine Glioma: From Preclinical to Clinical Studies.Pharmaceutics. 2022 Aug 24;14(9):1762. doi: 10.3390/pharmaceutics14091762. Pharmaceutics. 2022. PMID: 36145510 Free PMC article. Review.
-
Epidemiology, Diagnostic Strategies, and Therapeutic Advances in Diffuse Midline Glioma.J Clin Med. 2023 Aug 12;12(16):5261. doi: 10.3390/jcm12165261. J Clin Med. 2023. PMID: 37629304 Free PMC article. Review.
-
Generation of immunocompetent syngeneic allograft mouse models for pediatric diffuse midline glioma.Neurooncol Adv. 2022 May 24;4(1):vdac079. doi: 10.1093/noajnl/vdac079. eCollection 2022 Jan-Dec. Neurooncol Adv. 2022. PMID: 35733514 Free PMC article.
-
A robust immune-related gene pairs signature for predicting the overall survival of esophageal cancer.BMC Genomics. 2023 Jul 10;24(1):385. doi: 10.1186/s12864-023-09496-x. BMC Genomics. 2023. PMID: 37430202 Free PMC article.
-
IL-13Rα2 Status Predicts GB-13 (IL13.E13K-PE4E) Efficacy in High-Grade Glioma.Pharmaceutics. 2022 Apr 24;14(5):922. doi: 10.3390/pharmaceutics14050922. Pharmaceutics. 2022. PMID: 35631512 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

