Assessing risks of Plasmodium falciparum resistance to select next-generation antimalarials
- PMID: 34001441
- PMCID: PMC8282644
- DOI: 10.1016/j.pt.2021.04.006
Assessing risks of Plasmodium falciparum resistance to select next-generation antimalarials
Abstract
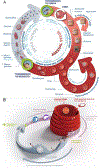
Strategies to counteract or prevent emerging drug resistance are crucial for the design of next-generation antimalarials. In the past, resistant parasites were generally identified following treatment failures in patients, and compounds would have to be abandoned late in development. An early understanding of how candidate therapeutics lose efficacy as parasites evolve resistance is important to facilitate drug design and improve resistance detection and monitoring up to the postregistration phase. We describe a new strategy to assess resistance to antimalarial compounds as early as possible in preclinical development by leveraging tools to define the Plasmodium falciparum resistome, predict potential resistance risks of clinical failure for candidate therapeutics, and inform decisions to guide antimalarial drug development.
Keywords: Plasmodium falciparum; drug discovery; malaria; resistance.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests M.D., J.N.B., T.N.C.W., and D.L. are employees of Medicines for Malaria Venture (Geneva). The remaining authors have no interests to declare.
Figures



References
-
- World Health Organization (2016) Global technical strategy for malaria 2016–2030, World Health Organization.
-
- Marapana D and Cowman AF (2020) Uncovering the ART of antimalarial resistance. Science 367, 22–23 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

