Nodosome Inhibition as a Novel Broad-Spectrum Antiviral Strategy against Arboviruses, Enteroviruses, and SARS-CoV-2
- PMID: 34001511
- PMCID: PMC8284462
- DOI: 10.1128/AAC.00491-21
Nodosome Inhibition as a Novel Broad-Spectrum Antiviral Strategy against Arboviruses, Enteroviruses, and SARS-CoV-2
Abstract
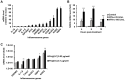
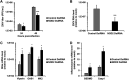
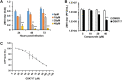
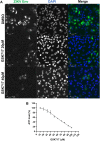
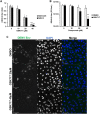
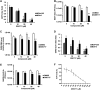
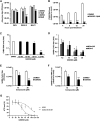
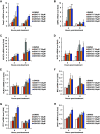
In the present report, we describe two small molecules with broad-spectrum antiviral activity. These drugs block the formation of the nodosome. The studies were prompted by the observation that infection of human fetal brain cells with Zika virus (ZIKV) induces the expression of nucleotide-binding oligomerization domain-containing protein 2 (NOD2), a host factor that was found to promote ZIKV replication and spread. A drug that targets NOD2 was shown to have potent broad-spectrum antiviral activity against other flaviviruses, alphaviruses, enteroviruses, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19). Another drug that inhibits receptor-interacting serine/threonine protein kinase 2 (RIPK2), which functions downstream of NOD2, also decreased the replication of these pathogenic RNA viruses. The antiviral effect of this drug was particularly potent against enteroviruses. The broad-spectrum action of nodosome-targeting drugs is mediated in part by the enhancement of the interferon response. Together, these results suggest that further preclinical investigation of nodosome inhibitors as potential broad-spectrum antivirals is warranted.
Keywords: COVID-19; NOD2; RIPK2; SARS-CoV-2; antiviral; arbovirus; broad spectrum; coxsackievirus; interferon; nodosome.
Figures
Similar articles
-
Broadly Active Antiviral Compounds Disturb Zika Virus Progeny Release Rescuing Virus-Induced Toxicity in Brain Organoids.Viruses. 2020 Dec 29;13(1):37. doi: 10.3390/v13010037. Viruses. 2020. PMID: 33383826 Free PMC article.
-
Bithiazole Inhibitors of Phosphatidylinositol 4-Kinase (PI4KIIIβ) as Broad-Spectrum Antivirals Blocking the Replication of SARS-CoV-2, Zika Virus, and Human Rhinoviruses.ChemMedChem. 2021 Dec 6;16(23):3548-3552. doi: 10.1002/cmdc.202100483. Epub 2021 Sep 7. ChemMedChem. 2021. PMID: 34382337 Free PMC article.
-
Viruses harness YxxØ motif to interact with host AP2M1 for replication: A vulnerable broad-spectrum antiviral target.Sci Adv. 2020 Aug 28;6(35):eaba7910. doi: 10.1126/sciadv.aba7910. eCollection 2020 Aug. Sci Adv. 2020. PMID: 32923629 Free PMC article.
-
Host Cell Targets for Unconventional Antivirals against RNA Viruses.Viruses. 2023 Mar 17;15(3):776. doi: 10.3390/v15030776. Viruses. 2023. PMID: 36992484 Free PMC article. Review.
-
Broad-Spectrum Antiviral Strategies and Nucleoside Analogues.Viruses. 2021 Apr 13;13(4):667. doi: 10.3390/v13040667. Viruses. 2021. PMID: 33924302 Free PMC article. Review.
Cited by
-
Zika virus targets human trophoblast stem cells and prevents syncytialization in placental trophoblast organoids.Nat Commun. 2023 Sep 8;14(1):5541. doi: 10.1038/s41467-023-41158-0. Nat Commun. 2023. PMID: 37684223 Free PMC article.
-
SARS-CoV-2/COVID-19 and its relationship with NOD2 and ubiquitination.Clin Immunol. 2022 May;238:109027. doi: 10.1016/j.clim.2022.109027. Epub 2022 May 2. Clin Immunol. 2022. PMID: 35513305 Free PMC article. Review.
-
NOD1, NOD2, and NLRC5 Receptors in Antiviral and Antimycobacterial Immunity.Vaccines (Basel). 2022 Sep 7;10(9):1487. doi: 10.3390/vaccines10091487. Vaccines (Basel). 2022. PMID: 36146565 Free PMC article. Review.
-
Cytoplasmic RNA sensors and their interplay with RNA-binding partners in innate antiviral response: theme and variations.RNA. 2022 Apr;28(4):449-477. doi: 10.1261/rna.079016.121. Epub 2022 Jan 14. RNA. 2022. PMID: 35031583 Free PMC article. Review.
-
Human pulmonary microvascular endothelial cells respond to DAMPs from injured renal tubular cells.Pulm Circ. 2024 Jul 3;14(3):e12379. doi: 10.1002/pul2.12379. eCollection 2024 Jul. Pulm Circ. 2024. PMID: 38962184 Free PMC article.
References
-
- Ianevski A, Zusinaite E, Kuivanen S, Strand M, Lysvand H, Teppor M, Kakkola L, Paavilainen H, Laajala M, Kallio-Kokko H, Valkonen M, Kantele A, Telling K, Lutsar I, Letjuka P, Metelitsa N, Oksenych V, Bjørås M, Nordbø SA, Dumpis U, Vitkauskiene A, Öhrmalm C, Bondeson K, Bergqvist A, Aittokallio T, Cox RJ, Evander M, Hukkanen V, Marjomaki V, Julkunen I, Vapalahti O, Tenson T, Merits A, Kainov D. 2018. Novel activities of safe-in-human broad-spectrum antiviral agents. Antiviral Res 154:174–182. 10.1016/j.antiviral.2018.04.016. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

