Moving beyond the constraints of chemistry via crystal structure discovery with isotropic multiwell pair potentials
- PMID: 34001591
- PMCID: PMC8166130
- DOI: 10.1073/pnas.2024034118
Moving beyond the constraints of chemistry via crystal structure discovery with isotropic multiwell pair potentials
Abstract
The rigid constraints of chemistry-dictated by quantum mechanics and the discrete nature of the atom-limit the set of observable atomic crystal structures. What structures are possible in the absence of these constraints? Here, we systematically crystallize one-component systems of particles interacting with isotropic multiwell pair potentials. We investigate two tunable families of pairwise interaction potentials. Our simulations self-assemble a multitude of crystal structures ranging from basic lattices to complex networks. Sixteen of the structures have natural analogs spanning all coordination numbers found in inorganic chemistry. Fifteen more are hitherto unknown and occupy the space between covalent and metallic coordination environments. The discovered crystal structures constitute targets for self-assembly and expand our understanding of what a crystal structure can look like.
Keywords: crystal structures; isotropic pair potentials; self-assembly.
Conflict of interest statement
The authors declare no competing interest.
Figures
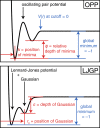


Comment in
- doi: 10.1073/pnas.2107024118
References
-
- Pauling L., The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry (Cornell University Press, 1960).
-
- Desiraju G. R., Crystal engineering: From molecule to crystal. J. Am. Chem. Soc. 135, 9952–9967 (2013). - PubMed
-
- Furukawa H., Cordova K. E., O’Keeffe M., Yaghi O. M., The chemistry and applications of metal-organic frameworks. Science 341, 1230444 (2013). - PubMed
-
- Boles M. A., Engel M., Talapin D. V., Self-assembly of colloidal nanocrystals: From intricate structures to functional materials. Chem. Rev. 116, 11220–11289 (2016). - PubMed
-
- Glotzer S. C., Solomon M. J., Anisotropy of building blocks and their assembly into complex structures. Nat. Mater. 6, 557–562 (2007). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

