Short hydrogen bonds enhance nonaromatic protein-related fluorescence
- PMID: 34001606
- PMCID: PMC8166056
- DOI: 10.1073/pnas.2020389118
Short hydrogen bonds enhance nonaromatic protein-related fluorescence
Abstract
Fluorescence in biological systems is usually associated with the presence of aromatic groups. Here, by employing a combined experimental and computational approach, we show that specific hydrogen bond networks can significantly affect fluorescence. In particular, we reveal that the single amino acid L-glutamine, by undergoing a chemical transformation leading to the formation of a short hydrogen bond, displays optical properties that are significantly enhanced compared with L-glutamine itself. Ab initio molecular dynamics simulations highlight that these short hydrogen bonds prevent the appearance of a conical intersection between the excited and the ground states and thereby significantly decrease nonradiative transition probabilities. Our findings open the door to the design of new photoactive materials with biophotonic applications.
Keywords: intrinsic fluorescence; short hydrogen bond; ultraviolet fluorescence.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

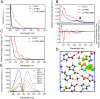


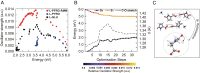
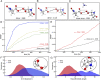
References
-
- Shukla A., et al. ., A novel UV laser-induced visible blue radiation from protein crystals and aggregates: Scattering artifacts or fluorescence transitions of peptide electrons delocalized through hydrogen bonding? Arch. Biochem. Biophys. 428, 144–153 (2004). - PubMed
-
- Amdursky N., et al. ., Blue luminescence based on quantum confinement at peptide nanotubes. Nano Lett. 9, 3111–3115 (2009). - PubMed
-
- Chan F. T., Pinotsi D., Schierle G. S. K., Kaminski C. F., Bio-nanoimaging: Protein Misfolding and Aggregation (Elsevier, 2013), pp. 147–155.
-
- Pinotsi D., et al. ., Proton transfer and structure-specific fluorescence in hydrogen bond-rich protein structures. J. Am. Chem. Soc. 138, 3046–3057 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

