Roles of KLF4 and AMPK in the inhibition of glycolysis by pulsatile shear stress in endothelial cells
- PMID: 34001623
- PMCID: PMC8166073
- DOI: 10.1073/pnas.2103982118
Roles of KLF4 and AMPK in the inhibition of glycolysis by pulsatile shear stress in endothelial cells
Abstract
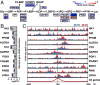
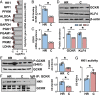
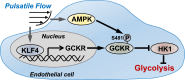
Vascular endothelial cells (ECs) sense and respond to hemodynamic forces such as pulsatile shear stress (PS) and oscillatory shear stress (OS). Among the metabolic pathways, glycolysis is differentially regulated by atheroprone OS and atheroprotective PS. Studying the molecular mechanisms by which PS suppresses glycolytic flux at the epigenetic, transcriptomic, and kinomic levels, we have demonstrated that glucokinase regulatory protein (GCKR) was markedly induced by PS in vitro and in vivo, although PS down-regulates other glycolysis enzymes such as hexokinase (HK1). Using next-generation sequencing data, we identified the binding of PS-induced Krüppel-like factor 4 (KLF4), which functions as a pioneer transcription factor, binding to the GCKR promoter to change the chromatin structure for transactivation of GCKR. At the posttranslational level, PS-activated AMP-activated protein kinase (AMPK) phosphorylates GCKR at Ser-481, thereby enhancing the interaction between GCKR and HK1 in ECs. In vivo, the level of phosphorylated GCKR Ser-481 and the interaction between GCKR and HK1 were increased in the thoracic aorta of wild-type AMPKα2+/+ mice in comparison with littermates with EC ablation of AMPKα2 (AMPKα2-/-). In addition, the level of GCKR was elevated in the aortas of mice with a high level of voluntary wheel running. The underlying mechanisms for the PS induction of GCKR involve regulation at the epigenetic level by KLF4 and at the posttranslational level by AMPK.
Keywords: AMPK; GCKR; KLF4; epigenetics; glycolysis.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
METTL3 mediates atheroprone flow-induced glycolysis in endothelial cells.Proc Natl Acad Sci U S A. 2025 May 13;122(19):e2424796122. doi: 10.1073/pnas.2424796122. Epub 2025 May 6. Proc Natl Acad Sci U S A. 2025. PMID: 40327688 Free PMC article.
-
Atheroprotective Flow Upregulates ITPR3 (Inositol 1,4,5-Trisphosphate Receptor 3) in Vascular Endothelium via KLF4 (Krüppel-Like Factor 4)-Mediated Histone Modifications.Arterioscler Thromb Vasc Biol. 2019 May;39(5):902-914. doi: 10.1161/ATVBAHA.118.312301. Arterioscler Thromb Vasc Biol. 2019. PMID: 30917677 Free PMC article.
-
Shear stress, SIRT1, and vascular homeostasis.Proc Natl Acad Sci U S A. 2010 Jun 1;107(22):10268-73. doi: 10.1073/pnas.1003833107. Epub 2010 May 17. Proc Natl Acad Sci U S A. 2010. PMID: 20479254 Free PMC article.
-
Mechanosensitive microRNAs-role in endothelial responses to shear stress and redox state.Free Radic Biol Med. 2013 Sep;64:61-8. doi: 10.1016/j.freeradbiomed.2013.05.034. Epub 2013 May 30. Free Radic Biol Med. 2013. PMID: 23727269 Free PMC article. Review.
-
Atherosclerosis and flow: roles of epigenetic modulation in vascular endothelium.J Biomed Sci. 2019 Aug 7;26(1):56. doi: 10.1186/s12929-019-0551-8. J Biomed Sci. 2019. PMID: 31387590 Free PMC article. Review.
Cited by
-
Silencing KRIT1 Partially Reverses the Effects of Disturbed Flow on the Endothelial Cell Transcriptome.Int J Mol Sci. 2025 May 2;26(9):4340. doi: 10.3390/ijms26094340. Int J Mol Sci. 2025. PMID: 40362576 Free PMC article.
-
Mechanobiology research in China.Mechanobiol Med. 2023 Jul 5;1(1):100002. doi: 10.1016/j.mbm.2023.100002. eCollection 2023 Sep. Mechanobiol Med. 2023. PMID: 40395869 Free PMC article. Review.
-
ASF1A-dependent P300-mediated histone H3 lysine 18 lactylation promotes atherosclerosis by regulating EndMT.Acta Pharm Sin B. 2024 Jul;14(7):3027-3048. doi: 10.1016/j.apsb.2024.03.008. Epub 2024 Mar 12. Acta Pharm Sin B. 2024. PMID: 39027248 Free PMC article.
-
Spontaneously Right-Side-Out-Orientated Coupling-Driven ROS-Sensitive Nanoparticles on Cell Membrane Inner Leaflet for Efficient Renovation in Vascular Endothelial Injury.Adv Sci (Weinh). 2023 Feb;10(6):e2205093. doi: 10.1002/advs.202205093. Epub 2023 Jan 26. Adv Sci (Weinh). 2023. PMID: 36703487 Free PMC article.
-
A Comparison of the Immunometabolic Effect of Antibiotics and Plant Extracts in a Chicken Macrophage-like Cell Line during a Salmonella Enteritidis Challenge.Antibiotics (Basel). 2023 Feb 8;12(2):357. doi: 10.3390/antibiotics12020357. Antibiotics (Basel). 2023. PMID: 36830268 Free PMC article.
References
-
- Doddaballapur A., et al. ., Laminar shear stress inhibits endothelial cell metabolism via KLF2-mediated repression of PFKFB3. Arterioscler. Thromb. Vasc. Biol. 35, 137–145 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

