One-Stop Serum Assay Identifies COVID-19 Disease Severity and Vaccination Responses
- PMID: 34001652
- PMCID: PMC9190970
- DOI: 10.4049/immunohorizons.2100011
One-Stop Serum Assay Identifies COVID-19 Disease Severity and Vaccination Responses
Abstract
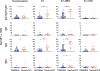
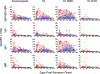
SARS-CoV-2 has caused over 100,000,000 cases and almost 2,500,000 deaths globally. Comprehensive assessment of the multifaceted antiviral Ab response is critical for diagnosis, differentiation of severity, and characterization of long-term immunity, especially as COVID-19 vaccines become available. Severe disease is associated with early, massive plasmablast responses. We developed a multiplex immunoassay from serum/plasma of acutely infected and convalescent COVID-19 patients and prepandemic and postpandemic healthy adults. We measured IgA, IgG, and/or IgM against SARS-CoV-2 nucleocapsid (N), spike domain 1 (S1), S1-receptor binding domain (RBD) and S1-N-terminal domain. For diagnosis, the combined [IgA + IgG + IgM] or IgG levels measured for N, S1, and S1-RBD yielded area under the curve values ≥0.90. Virus-specific Ig levels were higher in patients with severe/critical compared with mild/moderate infections. A strong prozone effect was observed in sera from severe/critical patients-a possible source of underestimated Ab concentrations in previous studies. Mild/moderate patients displayed a slower rise and lower peak in anti-N and anti-S1 IgG levels compared with severe/critical patients, but anti-RBD IgG and neutralization responses reached similar levels at 2-4 mo after symptom onset. Measurement of the Ab responses in sera from 18 COVID-19-vaccinated patients revealed specific responses for the S1-RBD Ag and none against the N protein. This highly sensitive, SARS-CoV-2-specific, multiplex immunoassay measures the magnitude, complexity, and kinetics of the Ab response and can distinguish serum Ab responses from natural SARS-CoV-2 infections (mild or severe) and mRNA COVID-19 vaccines.
Copyright © 2021 The Authors.
Conflict of interest statement
DISCLOSURES
F.E.-H.L. is the founder of Micro-B-plex, Inc. N.S.H., A.M.-P., and J.L.D. are also scientists at MicroB-plex, Inc., Atlanta, GA. A.-K.I.W. holds equity and management roles in Ataia Medical. The other authors have no financial conflicts of interest.
Figures




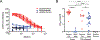

References
-
- Chan JF-W, Yip CC-Y, To KK-W, Tang TH-C, Wong SC-Y, Leung K-H, Fung AY-F, Ng AC-K, Zou Z, Tsoi H-W, et al. 2020. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J. Clin. Microbiol 58: e00310–20. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

