Intravitreal conbercept for diabetic macular oedema: 2-year results from a randomised controlled trial and open-label extension study
- PMID: 34001667
- PMCID: PMC9510409
- DOI: 10.1136/bjophthalmol-2020-318690
Intravitreal conbercept for diabetic macular oedema: 2-year results from a randomised controlled trial and open-label extension study
Abstract
Background: To demonstrate the efficacy and safety of intravitreal injections of conbercept versus laser photocoagulation in the treatment of diabetic macular oedema (DME).
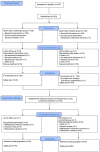
Methods: A 12-month multicentre, randomised, double-masked, double-sham, parallel controlled, phase III trial (Sailing Study), followed by a 12-month open-label extension study. Patients with centre-involved DME were randomly assigned to receive either laser photocoagulation followed by pro re nata (PRN) sham intravitreal injections (laser/sham) or sham laser photocoagulation followed by PRN 0.5 mg conbercept intravitreal injections (sham/conbercept). Patients who entered the extension study received PRN conbercept treatment. The primary endpoint was the changes in best-corrected visual acuity (BCVA) from baseline.
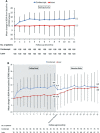
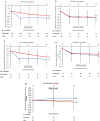
Results: A total of 248 eyes were included in the full analysis set and 157 eyes continued in the extension study. Significant improvement in mean change in BCVA from baseline to month 12 was observed in the sham/conbercept group (8.2±9.5 letters), whereas no improvement was observed in the laser/sham group (0.3±12.0 letters). Patients in the laser/sham group showed a marked improvement in BCVA after the switch to conbercept in the extension study, and there was no difference in BCVA between the two groups at the end of the extension study.
Conclusion: The use of a conbercept PRN intravitreal injection regimen improved the BCVA of patients with DME, and its efficacy was better than that of laser photocoagulations, and the same efficacy was observed when the eyes treated with laser alone were switched to conbercept.
Trial registration number: NCT02194634.
Keywords: clinical trial; macula; retina; treatment lasers.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: XK, QW, JL, ST and XW are employees of Chengdu Kanghong Biotechnology, Company, Ltd. PR and PK are consultants to Chengdu Kanghong Biotech.
Figures
References
-
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010;376:124–36. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
