Establishing Sickle Cell Disease Stroke Prevention Teams in Africa is Feasible: Program Evaluation Using the RE-AIM Framework
- PMID: 34001783
- PMCID: PMC8728755
- DOI: 10.1097/MPH.0000000000002179
Establishing Sickle Cell Disease Stroke Prevention Teams in Africa is Feasible: Program Evaluation Using the RE-AIM Framework
Erratum in
-
Establishing Sickle Cell Disease Stroke Prevention Teams in Africa is Feasible: Program Evaluation Using the RE-AIM Framework: Erratum.J Pediatr Hematol Oncol. 2022 May 1;44(4):193. doi: 10.1097/MPH.0000000000002471. Epub 2022 Apr 21. J Pediatr Hematol Oncol. 2022. PMID: 35452201 Free PMC article. No abstract available.
Abstract
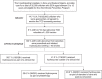
We used the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework to evaluate a Stroke Prevention Team's readiness to prevent strokes in children with sickle cell anemia living in northern Nigeria. The NIH sponsored Stroke Prevention Trial in Nigeria included a goal of a sustainable stroke prevention program. The program's 1-year reach for transcranial Doppler screening was 14.7% (4710/32,000) of which 6.0% (281/4710) had abnormal velocities (≥200 cm/s). All participants with abnormal transcranial Doppler velocities were started on hydroxyurea (effectiveness). The leaders of all 5 hospitals agreed to adopt the program. After 1 year, program-implementation and maintenance rates were 100%, demonstrating the program's feasibility and short-term sustainability.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
M.R.D.: had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. M.R.D.: designed the study. S.U.A. and M.A.T.: provided the TCD training. A.H.D., H.B.-M., H.I., A.T., A.A.S., B.W.J., S.G., Y.K., L.H., S.A., M.A.Z., and N.I.: administered the TCD screening. E.T. and L.J.: verified the neurological examinations. A.I.G., F.M.U., M.A.T., A.I.G., S.U.A., B.C.G., J.G., and D.L.G.: collected the data. D.L.G. and M.R.: performed the analyses. M.R.D., S.A., D.L.G., A.A.B., M.R.D.: interpreted the results. D.L.G., A.A.B., L.C.J., E.T., M.H.A., and M.R.D.: wrote the manuscript. Before submission, all authors reviewed the manuscript.The authors declare no conflict of interest.
Figures

References
-
- Kwiatkowski JL, Kanter J, Fullerton HJ, et al. Ischemic stroke in children and young adults with sickle cell disease (SCD) in the post-STOP era. Blood. 2015;126:68. - PubMed
-
- Kanter J, Kwiatkowski J, Fullerton HJ, et al. Impact of TCD screening protocol on the incidence of hemorrhagic stroke in children and young adults with sickle cell disease. Blood. 2015;126:3402.
-
- Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. - PubMed
-
- Kwiatkowski JL, Voeks JH, Kanter J, et al. Ischemic stroke in children and young adults with sickle cell disease in the post-STOP era. Am J Hematol. 2019;94:1335–1343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

