Parkin regulates drug-taking behavior in rat model of methamphetamine use disorder
- PMID: 34001858
- PMCID: PMC8129108
- DOI: 10.1038/s41398-021-01387-7
Parkin regulates drug-taking behavior in rat model of methamphetamine use disorder
Abstract
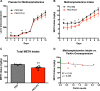
There is no FDA-approved medication for methamphetamine (METH) use disorder. New therapeutic approaches are needed, especially for people who use METH heavily and are at high risk for overdose. This study used genetically engineered rats to evaluate PARKIN as a potential target for METH use disorder. PARKIN knockout, PARKIN-overexpressing, and wild-type young adult male Long Evans rats were trained to self-administer high doses of METH using an extended-access METH self-administration paradigm. Reinforcing/rewarding properties of METH were assessed by quantifying drug-taking behavior and time spent in a METH-paired environment. PARKIN knockout rats self-administered more METH and spent more time in the METH-paired environment than wild-type rats. Wild-type rats overexpressing PARKIN self-administered less METH and spent less time in the METH-paired environment. PARKIN knockout rats overexpressing PARKIN self-administered less METH during the first half of drug self-administration days than PARKIN-deficient rats. The results indicate that rats with PARKIN excess or PARKIN deficit are useful models for studying neural substrates underlying "resilience" or vulnerability to METH use disorder and identify PARKIN as a novel potential drug target to treat heavy use of METH.
Conflict of interest statement
The authors declare no competing interests.
Figures
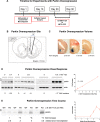
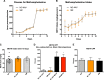
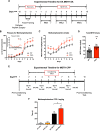

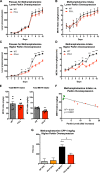
Similar articles
-
The Proteomic Landscape of Parkin-Deficient and Parkin-Overexpressing Rat Nucleus Accumbens: An Insight into the Role of Parkin in Methamphetamine Use Disorder.Biomolecules. 2025 Jul 3;15(7):958. doi: 10.3390/biom15070958. Biomolecules. 2025. PMID: 40723830 Free PMC article.
-
Parkin-deficient rats are resistant to neurotoxicity of chronic high-dose methamphetamine.Exp Neurol. 2021 Nov;345:113811. doi: 10.1016/j.expneurol.2021.113811. Epub 2021 Jul 21. Exp Neurol. 2021. PMID: 34298012
-
Overexpression of parkin in the rat nigrostriatal dopamine system protects against methamphetamine neurotoxicity.Exp Neurol. 2013 Sep;247:359-72. doi: 10.1016/j.expneurol.2013.01.001. Epub 2013 Jan 9. Exp Neurol. 2013. PMID: 23313192 Free PMC article.
-
Self-administration of methamphetamine alters gut biomarkers of toxicity.Eur J Neurosci. 2017 Aug;46(3):1918-1932. doi: 10.1111/ejn.13630. Eur J Neurosci. 2017. PMID: 28661099 Free PMC article.
-
Effect of Parkin on methamphetamine-induced α-synuclein degradation dysfunction in vitro and in vivo.Brain Behav. 2020 Apr;10(4):e01574. doi: 10.1002/brb3.1574. Epub 2020 Feb 21. Brain Behav. 2020. PMID: 32086884 Free PMC article.
Cited by
-
Exploring the progression of drug dependence in a methamphetamine self-administration rat model through targeted and non-targeted metabolomics analyses.Sci Rep. 2024 Sep 29;14(1):22543. doi: 10.1038/s41598-024-73247-5. Sci Rep. 2024. PMID: 39343795 Free PMC article.
-
An update: epigenetic mechanisms underlying methamphetamine addiction.Front Cell Dev Biol. 2024 Nov 22;12:1494557. doi: 10.3389/fcell.2024.1494557. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39650725 Free PMC article. Review.
-
Epigenetic mechanisms involved in methamphetamine addiction.Front Pharmacol. 2022 Aug 26;13:984997. doi: 10.3389/fphar.2022.984997. eCollection 2022. Front Pharmacol. 2022. PMID: 36091781 Free PMC article. Review.
-
The Proteomic Landscape of Parkin-Deficient and Parkin-Overexpressing Rat Nucleus Accumbens: An Insight into the Role of Parkin in Methamphetamine Use Disorder.Biomolecules. 2025 Jul 3;15(7):958. doi: 10.3390/biom15070958. Biomolecules. 2025. PMID: 40723830 Free PMC article.
-
Interactions of VMAT2 with CDCrel-1 and Parkin in Methamphetamine Neurotoxicity.Int J Mol Sci. 2024 Dec 5;25(23):13070. doi: 10.3390/ijms252313070. Int J Mol Sci. 2024. PMID: 39684782 Free PMC article.
References
-
- NIDA. Overdose Death Rates. https://www.drugabuse.gov/drug-topics/trends-statistics/overdose-death-r.... (2018).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

