TRPM7 kinase-mediated immunomodulation in macrophage plays a central role in magnesium ion-induced bone regeneration
- PMID: 34001887
- PMCID: PMC8128914
- DOI: 10.1038/s41467-021-23005-2
TRPM7 kinase-mediated immunomodulation in macrophage plays a central role in magnesium ion-induced bone regeneration
Abstract
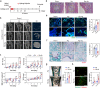
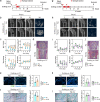
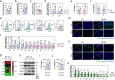
Despite the widespread observations on the osteogenic effects of magnesium ion (Mg2+), the diverse roles of Mg2+ during bone healing have not been systematically dissected. Here, we reveal a previously unknown, biphasic mode of action of Mg2+ in bone repair. During the early inflammation phase, Mg2+ contributes to an upregulated expression of transient receptor potential cation channel member 7 (TRPM7), and a TRPM7-dependent influx of Mg2+ in the monocyte-macrophage lineage, resulting in the cleavage and nuclear accumulation of TRPM7-cleaved kinase fragments (M7CKs). This then triggers the phosphorylation of Histone H3 at serine 10, in a TRPM7-dependent manner at the promoters of inflammatory cytokines, leading to the formation of a pro-osteogenic immune microenvironment. In the later remodeling phase, however, the continued exposure of Mg2+ not only lead to the over-activation of NF-κB signaling in macrophages and increased number of osteoclastic-like cells but also decelerates bone maturation through the suppression of hydroxyapatite precipitation. Thus, the negative effects of Mg2+ on osteogenesis can override the initial pro-osteogenic benefits of Mg2+. Taken together, this study establishes a paradigm shift in the understanding of the diverse and multifaceted roles of Mg2+ in bone healing.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats.Nat Med. 2016 Oct;22(10):1160-1169. doi: 10.1038/nm.4162. Epub 2016 Aug 29. Nat Med. 2016. PMID: 27571347 Free PMC article.
-
TRPM7 and MagT1 in the osteogenic differentiation of human mesenchymal stem cells in vitro.Sci Rep. 2018 Nov 1;8(1):16195. doi: 10.1038/s41598-018-34324-8. Sci Rep. 2018. PMID: 30385806 Free PMC article.
-
A Biodegradable Mg-Based Alloy Inhibited the Inflammatory Response of THP-1 Cell-Derived Macrophages Through the TRPM7-PI3K-AKT1 Signaling Axis.Front Immunol. 2019 Dec 3;10:2798. doi: 10.3389/fimmu.2019.02798. eCollection 2019. Front Immunol. 2019. PMID: 31849975 Free PMC article.
-
TRPM7 and its role in neurodegenerative diseases.Channels (Austin). 2015;9(5):253-61. doi: 10.1080/19336950.2015.1075675. Epub 2015 Jul 28. Channels (Austin). 2015. PMID: 26218331 Free PMC article. Review.
-
Immunomodulatory functions of TRPM7 and its implications in autoimmune diseases.Immunology. 2022 Jan;165(1):3-21. doi: 10.1111/imm.13420. Epub 2021 Oct 5. Immunology. 2022. PMID: 34558663 Review.
Cited by
-
Enhancing the immunomodulatory osteogenic properties of Ti-Mg alloy by Mg2+-containing nanostructures.Regen Biomater. 2024 Aug 29;11:rbae104. doi: 10.1093/rb/rbae104. eCollection 2024. Regen Biomater. 2024. PMID: 39372848 Free PMC article.
-
Bioactive elements manipulate bone regeneration.Biomater Transl. 2023 Dec 28;4(4):248-269. doi: 10.12336/biomatertransl.2023.04.005. eCollection 2023. Biomater Transl. 2023. PMID: 38282709 Free PMC article. Review.
-
A comparative study on the effects of biodegradable high-purity magnesium screw and polymer screw for fixation in epiphyseal trabecular bone.Regen Biomater. 2024 Sep 3;11:rbae095. doi: 10.1093/rb/rbae095. eCollection 2024. Regen Biomater. 2024. PMID: 39346687 Free PMC article.
-
Feasibility and Efficacy of a Degradable Magnesium-Alloy GBR Membrane for Bone Augmentation in a Distal Bone-Defect Model in Beagle Dogs.Bioinorg Chem Appl. 2022 Mar 23;2022:4941635. doi: 10.1155/2022/4941635. eCollection 2022. Bioinorg Chem Appl. 2022. PMID: 35371192 Free PMC article.
-
Immunological reaction to magnesium-based implants for orthopedic applications. What do we know so far? A systematic review on in vivo studies.Mater Today Bio. 2022 Jun 9;15:100315. doi: 10.1016/j.mtbio.2022.100315. eCollection 2022 Jun. Mater Today Bio. 2022. PMID: 35757033 Free PMC article. Review.
References
-
- Salimi MH, Heughebaert JC, Nancollas GH. Crystal-growth of calcium phosphates in the presence of magnesium-ions. Langmuir. 1985;1:119–122. doi: 10.1021/la00061a019. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

