Net decrease in spine-surface GluA1-containing AMPA receptors after post-learning sleep in the adult mouse cortex
- PMID: 34001888
- PMCID: PMC8129120
- DOI: 10.1038/s41467-021-23156-2
Net decrease in spine-surface GluA1-containing AMPA receptors after post-learning sleep in the adult mouse cortex
Abstract
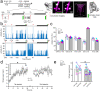
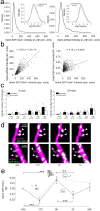
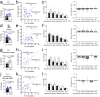
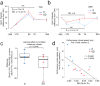
The mechanisms by which sleep benefits learning and memory remain unclear. Sleep may further strengthen the synapses potentiated by learning or promote broad synaptic weakening while protecting the newly potentiated synapses. We tested these ideas by combining a motor task whose consolidation is sleep-dependent, a marker of synaptic AMPA receptor plasticity, and repeated two-photon imaging to track hundreds of spines in vivo with single spine resolution. In mouse motor cortex, sleep leads to an overall net decrease in spine-surface GluA1-containing AMPA receptors, both before and after learning. Molecular changes in single spines during post-learning sleep are correlated with changes in performance after sleep. The spines in which learning leads to the largest increase in GluA1 expression have a relative advantage after post-learning sleep compared to sleep deprivation, because sleep weakens all remaining spines. These results are obtained in adult mice, showing that sleep-dependent synaptic down-selection also benefits the mature brain.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Effects of non-rapid eye movement sleep on the cortical synaptic expression of GluA1-containing AMPA receptors.Eur J Neurosci. 2024 Jul;60(2):3961-3972. doi: 10.1111/ejn.16460. Epub 2024 Jul 8. Eur J Neurosci. 2024. PMID: 38973508 Free PMC article.
-
Cortical Synaptic AMPA Receptor Plasticity during Motor Learning.Neuron. 2020 Mar 4;105(5):895-908.e5. doi: 10.1016/j.neuron.2019.12.005. Epub 2019 Dec 31. Neuron. 2020. PMID: 31901303 Free PMC article.
-
REM sleep selectively prunes and maintains new synapses in development and learning.Nat Neurosci. 2017 Mar;20(3):427-437. doi: 10.1038/nn.4479. Epub 2017 Jan 16. Nat Neurosci. 2017. PMID: 28092659 Free PMC article.
-
Structural modulation of dendritic spines during synaptic plasticity.Neuroscientist. 2012 Aug;18(4):326-41. doi: 10.1177/1073858411407206. Epub 2011 Jun 13. Neuroscientist. 2012. PMID: 21670426 Review.
-
Mechanisms of motor learning mediated by synaptic plasticity in rat primary motor cortex.Neurosci Res. 2018 Mar;128:14-18. doi: 10.1016/j.neures.2017.09.008. Epub 2017 Sep 23. Neurosci Res. 2018. PMID: 28951322 Review.
Cited by
-
Ultrastructural effects of sleep and wake on the parallel fiber synapses of the cerebellum.Elife. 2022 Dec 28;11:e84199. doi: 10.7554/eLife.84199. Elife. 2022. PMID: 36576248 Free PMC article.
-
The emergence of molecular systems neuroscience.Mol Brain. 2022 Jan 4;15(1):7. doi: 10.1186/s13041-021-00885-5. Mol Brain. 2022. PMID: 34983613 Free PMC article. Review.
-
Increased glutamatergic synaptic transmission during development in layer II/III mouse motor cortex pyramidal neurons.Cereb Cortex. 2023 Apr 4;33(8):4645-4653. doi: 10.1093/cercor/bhac368. Cereb Cortex. 2023. PMID: 36137566 Free PMC article.
-
Multi-region processing during sleep for memory and cognition.Proc Jpn Acad Ser B Phys Biol Sci. 2025;101(3):107-128. doi: 10.2183/pjab.101.008. Proc Jpn Acad Ser B Phys Biol Sci. 2025. PMID: 40074337 Free PMC article. Review.
-
Linking activity dyshomeostasis and sleep disturbances in Alzheimer disease.Nat Rev Neurosci. 2024 Apr;25(4):272-284. doi: 10.1038/s41583-024-00797-y. Epub 2024 Feb 19. Nat Rev Neurosci. 2024. PMID: 38374463 Review.
References
-
- Della Monica C, Johnsen S, Atzori G, Groeger JA, Dijk DJ. Rapid eye movement sleep, sleep continuity and slow wave sleep as predictors of cognition, mood, and subjective sleep quality in healthy men and women, aged 20-84 years. Front. Psychiatry. 2018;9:255. doi: 10.3389/fpsyt.2018.00255. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

